Purification process of N, O-1, 3-diacetyl indole
A technology of diacetyl indole and diacetyl indole, which is applied in the field of purification technology of N,O-1,3-diacetyl indole, can solve large waste solid pollution, nonconformity, column chromatography Difficulty in operation and other problems, to ensure product quality, avoid time-consuming, and avoid a lot of silica waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
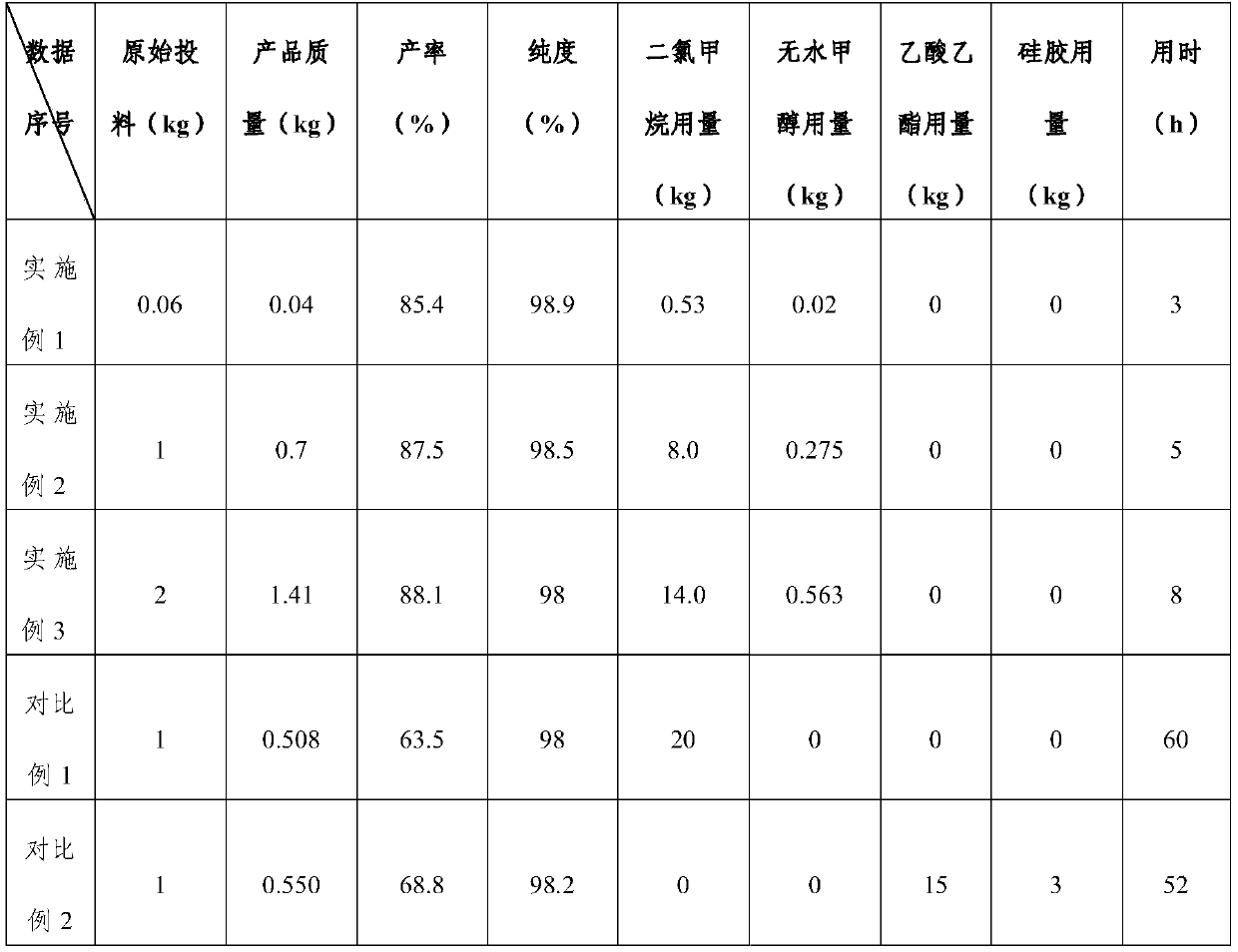
[0026] The crude product of N,O-1,3-diacetylindole with a purity of 80% is used as a raw material, and the purification process of a kind of N,O-1,3-diacetylindole of the present invention is adopted for purification, which comprises the following steps: Follow the steps below:
[0027]S1: Pretreatment, place the three-necked flask in an ultrasonic constant temperature water bath, start the stirrer, add 400 g of dichloromethane into the three-necked flask, and control the temperature of dichloromethane to be 30±2°C, add 60 g of N to the three-necked flask , The crude product of O-1,3-diacetyl indole was stirred for 30min, the three-necked flask was placed in a refrigerator of -18°C for 30min, and filtered to obtain the crude product of N,O-1,3-diacetyl indole 43g and primary filtrate;
[0028] S2: configure a slurry, mix dichloromethane and anhydrous methanol according to a volume ratio of 7:1, stir evenly to obtain a slurry, and the polarity of the slurry is 6 to 6.2;
[00...
Embodiment 2
[0033] The difference between Example 2 and Example 1 is that in the step S1, the N,O-1,3-diacetyl indole crude product input amount is 1kg, and the methylene chloride addition is 6kg to obtain N,O-1 , 720g of 3-diacetyl indole primary product; in the step S2, the added amount of the slurry is 2.2kg; in the step S4, the low-temperature drying temperature is 5°C, to obtain a white N,O-1 with a purity of 98.5%, The finished product of 3-diacetyl indole is 700g, and the total time is 5h.
Embodiment 3
[0035] The difference between Example 3 and Example 1 is that in the step S1, the N,O-1,3-diacetyl indole crude product input amount is 2kg, and the methylene chloride addition is 10kg to obtain N,O-1 , 1.5kg of the first product of 3-diacetyl indole; in the step S2, the added amount of the slurry is 4.5kg; in the step S4, the low-temperature drying temperature is 0° C. to obtain a white N,O-1 with a purity of 98%. ,3-Diacetyl indole finished product is 1.41kg, and the total time is 8h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com