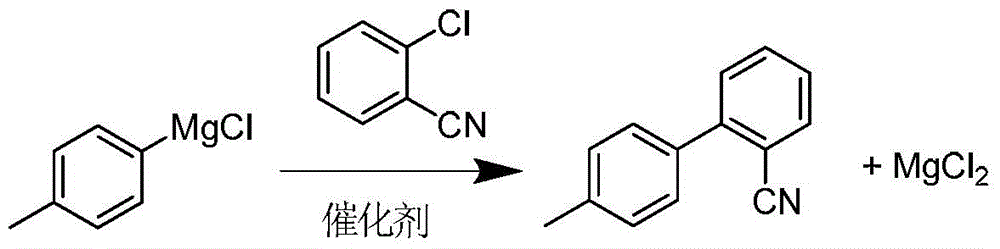
Cleaner production process of 2‑cyano‑4′‑methylbiphenyl
A technology of methyl biphenyl and production process, which is applied in the purification/separation of carboxylic acid nitrile, magnesium chloride, magnesium halide, etc., which can solve the problems of high solvent recovery cost and inability to discharge waste water, and achieve resource utilization and reduce treatment costs , Improve the effect of recycling efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Preparation of crude magnesium chloride: 20Kg content of 30% p-methylphenylmagnesium chloride tetrahydrofuran solution is slowly added dropwise at 0~10°C to a mixture containing 5.5Kg o-chlorobenzonitrile, 50g anhydrous ferric chloride and 14.5Kg tetrahydrofuran The solution was incubated for 3 hours to obtain a Grignard coupling reaction solution. Slowly add 2Kg tap water dropwise to the reaction solution at 20-25°C (apply the reclaimed aqueous tetrahydrofuran and tap water in batches, and ensure that the total amount of water added is 2Kg). Kilograms of magnesium chloride crude product and 36Kg filtrate.
[0025] (2) Purification of crude magnesium chloride: add 3 kg of tap water and 300 g of concentrated hydrochloric acid to 6 kg of crude magnesium chloride, adjust the pH to 6-7, and heat up to 60-70°C to dissolve all the solids. 2Kg of toluene was added to carry out extraction and layering to obtain about 2.3Kg of toluene solution in the upper layer. Concentra...
Embodiment 2
[0030] (1) Preparation of crude magnesium chloride: 20Kg content of 30% p-methylphenylmagnesium chloride tetrahydrofuran solution is slowly added dropwise at 0~10°C to a mixture containing 5.5Kg o-chlorobenzonitrile, 50g anhydrous ferric chloride and 14.5Kg tetrahydrofuran The solution was incubated for 3 hours to obtain a Grignard coupling reaction solution. Slowly add 3Kg tap water dropwise to the reaction solution at 20~25°C (apply the reclaimed aqueous tetrahydrofuran and tap water in batches, and ensure that the total amount of water added is 3Kg). Kilograms of magnesium chloride crude product and 36Kg filtrate.
[0031] (2) Purification of crude magnesium chloride: add 2 kg of tap water and 300 g of concentrated hydrochloric acid to 7 kg of crude magnesium chloride, adjust the pH to 6-7, and heat up to 60-70°C to dissolve all the solids. 2Kg of toluene was added to carry out extraction and layering to obtain about 2.3Kg of toluene solution in the upper layer. Concentra...
Embodiment 3
[0036](1) Preparation of crude magnesium chloride: 20Kg content of 24.3% p-methylphenylmagnesium chloride tetrahydrofuran solution is slowly added dropwise to a mixture containing 4.5Kg o-chlorobenzonitrile, 40g anhydrous ferric chloride and 14.5Kg tetrahydrofuran at 0-10°C. The solution was incubated for 4 hours to obtain a Grignard coupling reaction solution. Slowly add 1.75Kg tap water dropwise to the reaction solution at 20-25°C (apply the recovered aqueous tetrahydrofuran and tap water in batches, and ensure that the total amount of water added is 1.75Kg). Obtained 4.8 kg of magnesium chloride crude product and 36Kg of filtrate.
[0037] (2) Purification of crude magnesium chloride: add 1.6 kg of tap water and 240 g of concentrated hydrochloric acid to 4.8 kg of crude magnesium chloride, adjust the pH to 6-7, and heat up to 60-70°C to dissolve all the solids. Add 1.6Kg of toluene to carry out extraction and stratification to obtain about 1.8Kg of upper layer toluene solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com