Recombinant bacteria for producing carboxylesterase and application thereof
A carboxylesterase and carboxylester-producing technology, which is applied in the field of enzyme engineering, can solve the problem that the enzyme activity of carboxylesterase cannot meet industrial needs and other problems, and achieves the effect of efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Construction of engineering strains
[0040] The artificially synthesized carboxylesterase BaCEs02 gene sequence whose nucleotide sequence is shown in SEQ ID NO.2 (amino acid sequence is shown in SEQ ID NO.1). The BaCEs02 gene sequence and the plasmid vector pColdII were digested with restriction endonucleases SacI and XbaI and then ligated and transformed into E.coli BL21 (DE3) competent cells to obtain the recombinant strain E.coli BL21-pColdII-BaCEs02.
Embodiment 2
[0041] Embodiment 2: Expression and purification of carboxylesterase (BaCEs02)
[0042] LB medium g / L: sodium chloride 10, tryptone 10, Yeast Extract 5, pH 7.
[0043] Inoculate the recombinant Escherichia coli E.coli BL21-pColdII-BaCEs02 in a solution containing 100mg·mL -1In the LB liquid medium of ampicillin, the original strain E.coli BL21(DE3) and the empty strain (E.coli BL21(DE3) was transformed into the pClodII plasmid) were used as controls, cultured at 37°C, 200rmp for 12h, and then 500μL of the above The seed solution was inoculated in 50 mL LB medium containing 50 μL ampicillin, and cultured at 37 ° C for 2.5 h until the OD 600 to 0.6, cool the shaker to 15°C and let it stand for 30 minutes. 40 μL of IPTG with a final concentration of 0.4 mol / L was added to each bottle as an inducer, and no inducer was used as a control group, and cultured at 15°C and 200 rpm for 24 hours.
[0044] Collect the bacterial liquid, centrifuge at 4°C, 8000rmp for 10min to obtain the ...
Embodiment 3
[0045] Embodiment 3: the enzyme activity assay of carboxylesterase (BaCEs02)
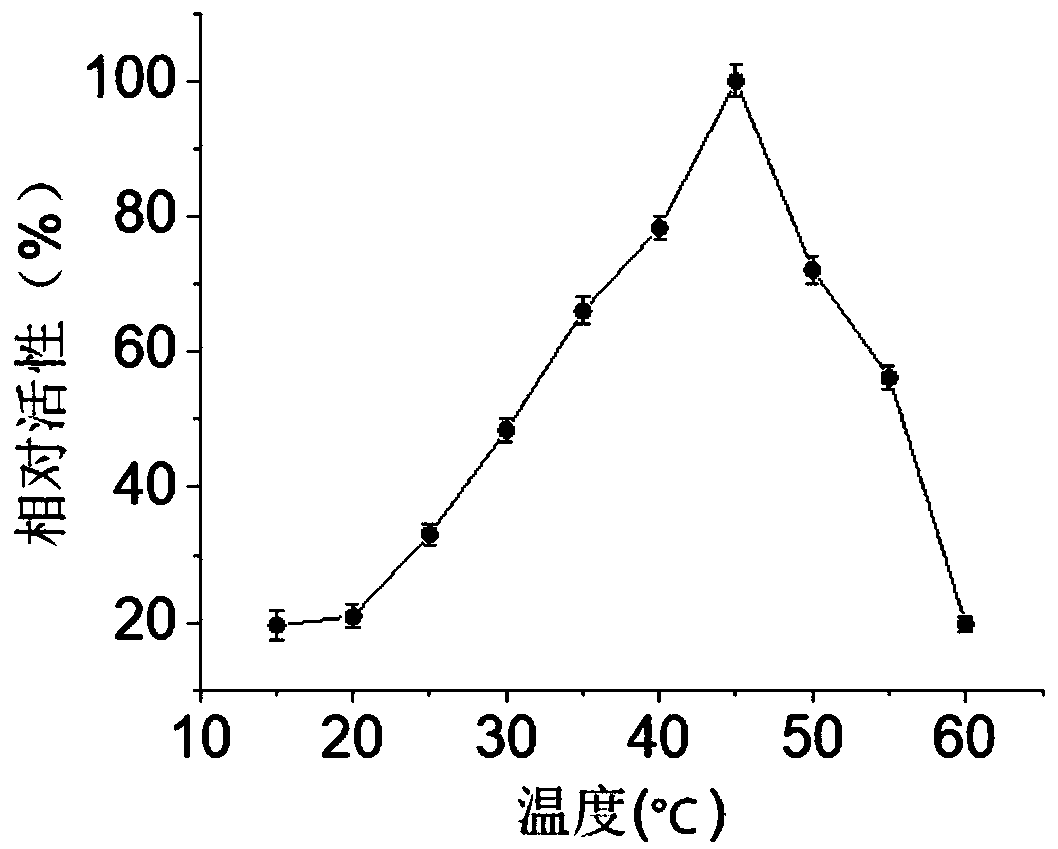
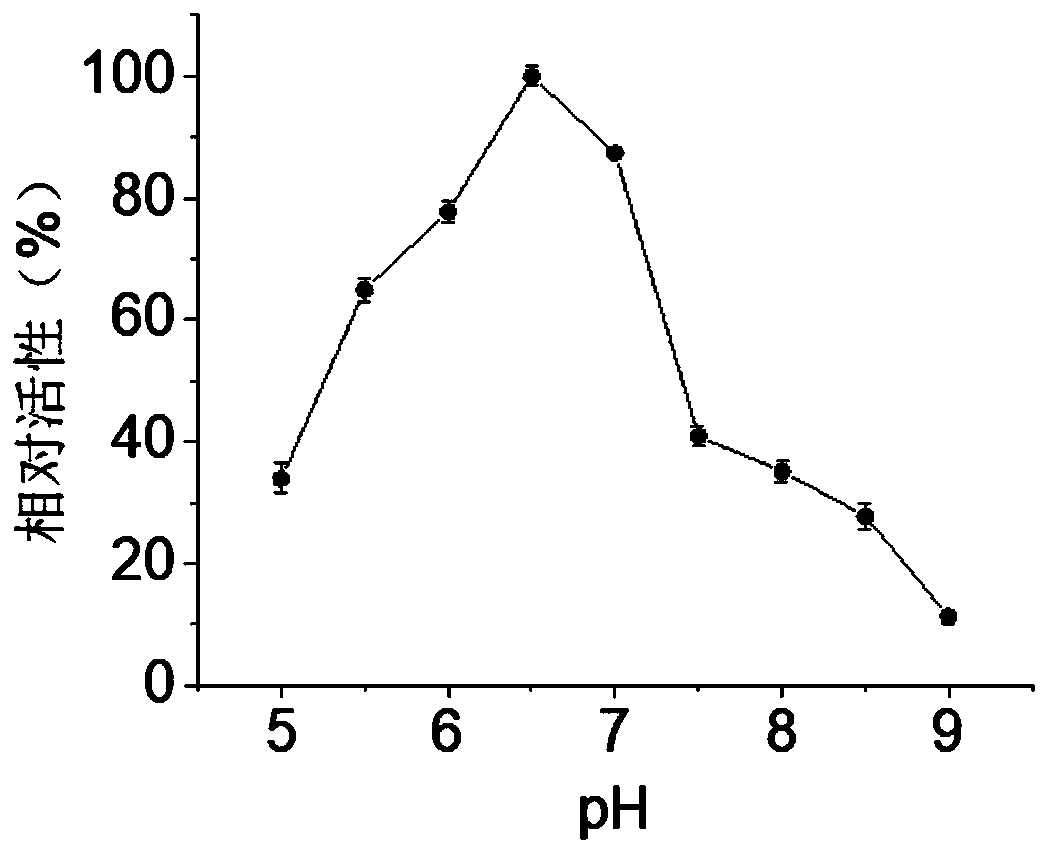
[0046] In disodium hydrogen phosphate-potassium dihydrogen phosphate buffer (pH 7), with 2-naphthyl acetate as substrate, in the range of 15 to 60°C, every 5°C, measure the enzyme activity of carboxylesterase BaCEs02, it can be known The optimum temperature for carboxylesterase BaCEs02 is 45°C (see figure 1 ). Under the optimum reaction temperature of 45°C, within the range of pH 5.0 to 8.0, measure the enzyme activity every 0.5 to determine the optimum reaction pH as 6.5 (see figure 2 ).
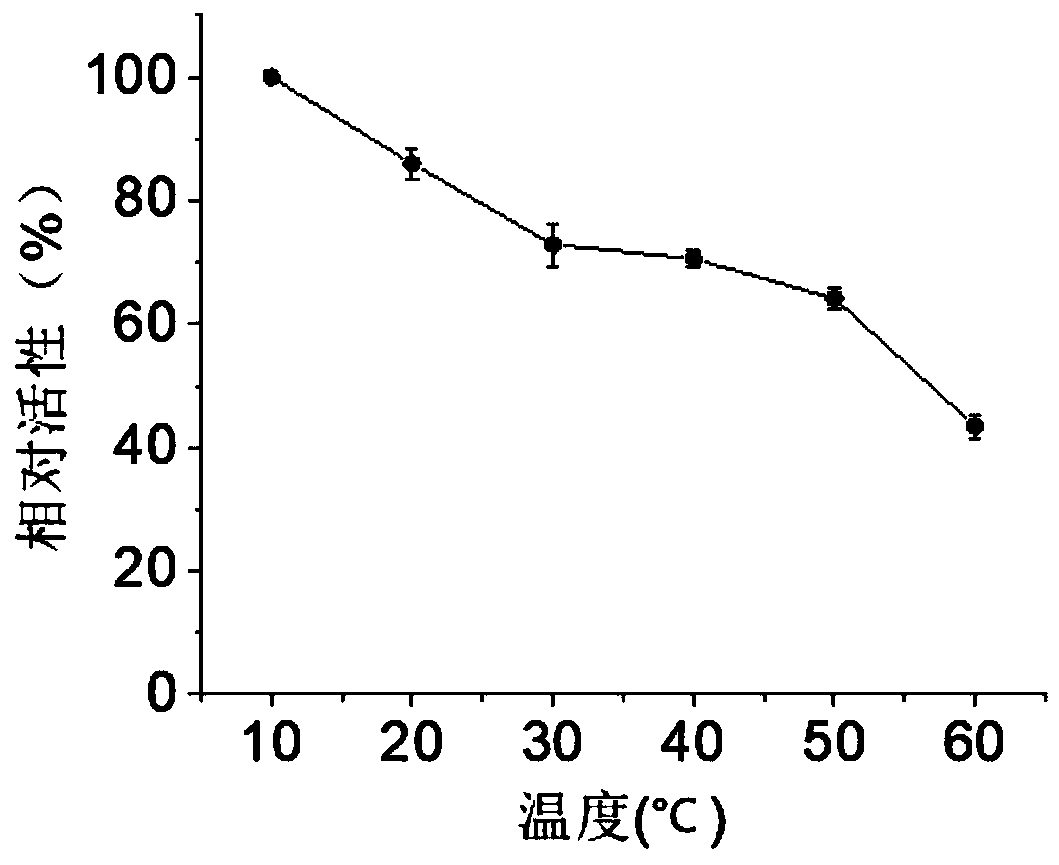
[0047] Under optimum reaction conditions, i.e. in disodium hydrogen phosphate-potassium dihydrogen phosphate buffer solution (pH 6.5), at 45°C, 0.6M 1-naphthyl acetate and 2-naphthyl acetate were used as substrates to determine the examples The enzyme activity of the crude enzyme liquid obtained in 2 and the specific enzyme activity of the carboxylesterase BaCEs02 obtained by purification, wherein the enzyme ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com