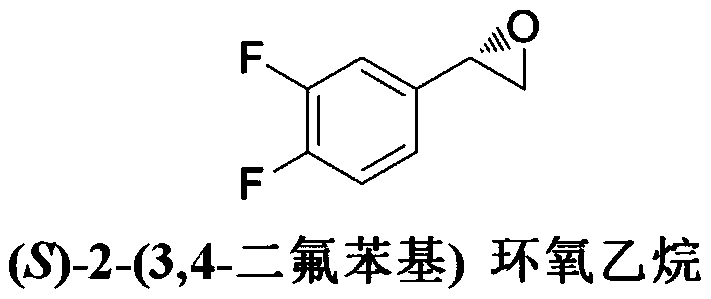
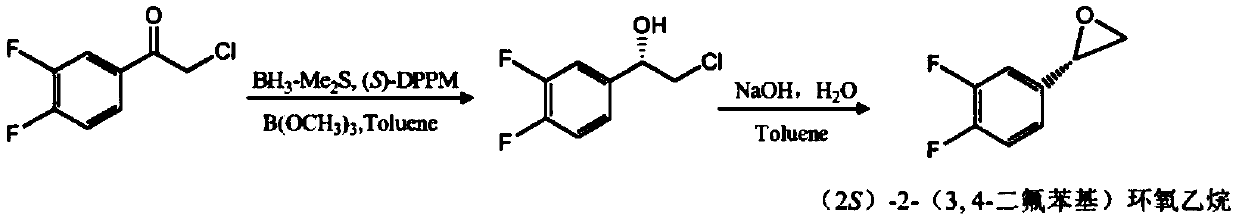
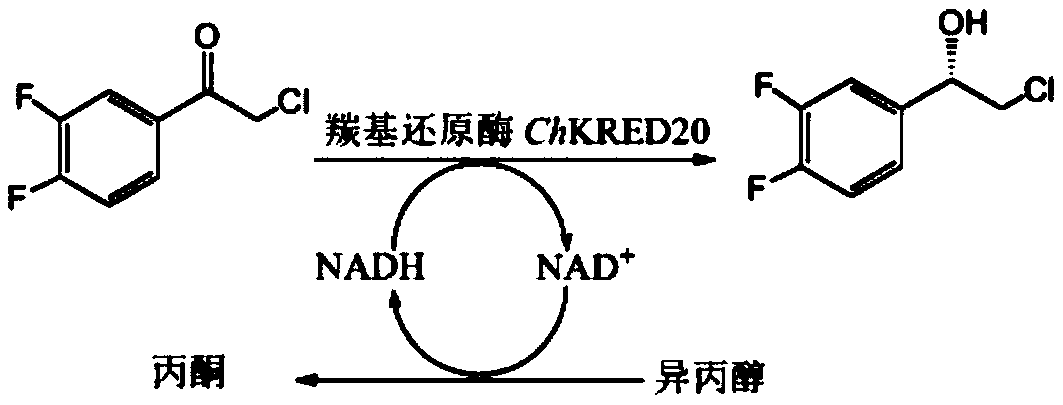
Method for preparing (S)-2-(3,4-difluorophenyl)ethylene oxide
A technology of difluorophenyl and oxirane is applied in the field of preparing chiral compound-2-oxirane, which can solve the problems of long reaction period, complicated post-processing and high cost, and achieves low cost and post-processing process. Simple, short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add N-methylmorpholine nitrogen oxide (16.2g, 2.3equiv., 138mmol), catalyst 1 (1.76g, 0.04equiv., 2.4mmol), 80mL of dichloromethane and 30mL of ethanol into a 250mL three-neck round bottom flask, Add 3,4-difluorostyrene (8.4g, 1.0equiv., 60.0mmol), stir at room temperature for 15 minutes, then cool to -45°C, start to slowly add 45mL of 85% m-chloroperoxybenzoic acid dissolved in ethanol dropwise (19.5g, 1.6equiv., 96.0mmol), the dropwise addition was completed in about half an hour, kept stirring for 4 hours, adjusted the pH to about 9 with 1.0mol / L sodium hydroxide solution, added 100mL dichloromethane and 15mL water for extraction , the organic layer was dried with anhydrous sodium sulfate for 3 hours, and the solvent was recovered under reduced pressure to obtain an orange viscous substance, which was obtained by column chromatography (eluent: petroleum ether / ethyl acetate=20 / 1) to obtain a yellow oily substance (S) -2-(3,4-difluorophenyl)oxirane 7.8g, yield 83%, e.e...
Embodiment 2
[0047]Add N-methylmorpholine nitrogen oxide (16.2g, 2.3equiv., 138mmol), catalyst 1 (1.76g, 0.04equiv., 2.4mmol), 80mL of dichloromethane and 30mL of ethanol into a 250mL three-neck round bottom flask, Add 3,4-difluorostyrene (8.4g, 1.0equiv., 60.0mmol), stir at room temperature for 15 minutes, then cool to -65°C, start to slowly add 45mL of 85% m-chloroperoxybenzoic acid dissolved in ethanol dropwise (19.5g, 1.6equiv., 96.0mmol), the dropwise addition was completed in about half an hour, kept stirring for 4 hours, adjusted the pH to about 9 with 1.0mol / L sodium hydroxide solution, added 100mL dichloromethane and 15mL water for extraction , the organic layer was dried with anhydrous sodium sulfate for 3 hours, and the solvent was reclaimed under reduced pressure to obtain an orange viscous substance, and a yellow oily substance ((S )-2-(3,4-difluorophenyl)oxirane) 7.1 g, yield 76%, e.e. value 91%.
Embodiment 3
[0049] Add N-methylmorpholine nitrogen oxide (16.2g, 2.3equiv., 138mmol), catalyst 3 (1.52g, 0.04equiv., 2.4mmol), 80mL of dichloromethane and 30mL of ethanol into a 250mL three-neck round bottom flask, Add 3,4-difluorostyrene (8.4g, 1.0equiv., 60.0mmol), stir at room temperature for 15 minutes, then cool to -45°C, start to slowly add 45mL of 85% m-chloroperoxybenzoic acid dissolved in ethanol dropwise (19.5g, 1.6equiv., 96.0mmol), the dropwise addition was completed in about half an hour, kept stirring for 4 hours, adjusted the pH to about 9 with 1.0mol / L sodium hydroxide solution, added 100mL dichloromethane and 15mL water for extraction , the organic layer was dried with anhydrous sodium sulfate for 3 hours, and the solvent was reclaimed under reduced pressure to obtain an orange viscous substance, and a yellow oily substance ((S )-2-(3,4-difluorophenyl)oxirane) 7.6 g, yield 81%, e.e. value 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com