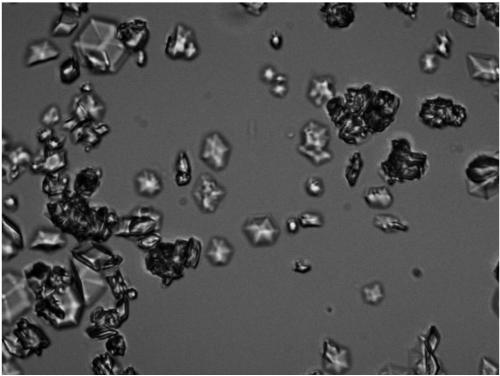
Preparation method for glycine-lysine-proline insulin crystals
A technology for insulin lispro and insulin, which is applied in the field of preparation of human insulin analog crystals, and can solve the problems of inability to form crystals of insulin lispro, lower safety, lower stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of recombinant insulin lispro crystals is carried out in the following four main steps:
[0065] 1) Prepare recombinant insulin lispro crystallization liquid at 25°C, the components and concentrations contained in the crystallization liquid are: recombinant insulin lispro: 15g / L; ethanol: 15%; acetic acid: 1.0M; sodium chloride : 0.3M; Phenol: 0.1% (mass volume ratio).
[0066] The preparation process is as follows: Weigh 1.5g of recombinant insulin lispro dry powder, measure 15ml of ethanol, 5.7ml of acetic acid, 1ml of 10% phenol, add 1.75g of sodium chloride, add water and mix to finally dissolve to 100ml to make a crystallization solution.
[0067] 2) Add 15.69 mg of zinc chloride (0.5% Zn ion) to the crystallization solution in step 1), and adjust the pH to 8.3 with ammonium hydroxide to make the crystallization solution completely clear.
[0068] 3) Then adjust the pH to 7.4 with acetic acid, stir at a speed of 1500 rpm, and adjust the temperatu...
Embodiment 2
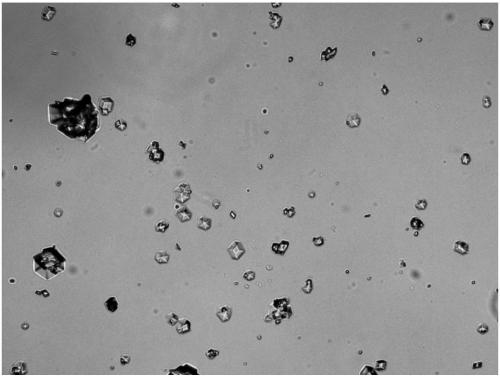
[0072] Steps 1)-4) of Example 1 were used to prepare recombinant insulin lispro crystals, except that the amount of Zn ions added in step 2) was 0.1%. After the crystallization was over, the supernatant was taken for HPLC detection, and the content of recombinant insulin lispro in the supernatant was 0.27 mg / ml; the crystal suspension was stirred up and examined under a microscope, and 160 times magnification showed obvious hexahedral crystals with relatively high transparency. high (see figure 2 ); the zinc content of the finally obtained crystalline product is 0.53%; the crystallization yield is 98.2%.
Embodiment 3
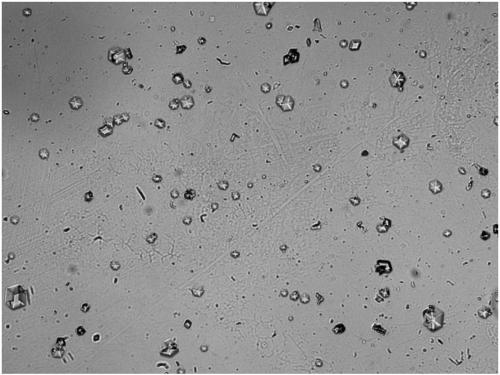
[0074] Steps 1)-4) of Example 1 were used to prepare recombinant insulin lispro crystals, except that the amount of Zn ions added in step 2) was 0.8%. After the crystallization was over, the supernatant was taken for HPLC detection, and the content of recombinant insulin lispro in the supernatant was 0.26 mg / ml; the crystal suspension was stirred up and examined under a microscope, and 160 times magnification showed obvious hexahedral crystals with relatively high transparency. high (see image 3 ); the zinc content of the finally obtained crystalline product is 0.81%; the crystallization yield is 98.27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com