a mn 4+ Preparation method of ion-activated fluoride phosphor
A fluorescent powder and fluoride technology, which is applied in the field of preparation of fluoride fluorescent powder, can solve the problems of difficult control of the preparation process, unsuitability for industrial production, and volatilization of hydrogen fluoride gas, and achieve the goals of short time consumption, convenient synthesis, and reduced dosage requirements Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: K 2 MnF 6 preparation of
[0045] Prepare K according to the method described in the literature Angew. 2 MnF 6 crystals.
[0046] 0.45g KMnO 4 and 9g KHF 2 Dissolve in 30mL hydrofluoric acid (49%), stir for 20 minutes, then gradually drop into about 1.2mL hydrogen peroxide (30wt.%), gradually form a yellow precipitate in the solution, filter the solution to obtain the precipitate, wash with acetone Bake at 60°C for 2 hours to get K 2 MnF 6 .
Embodiment 2
[0047] Example 2: K 2 NaAlF 6 :Mn 4+ Preparation of Fluoride Phosphor Powder
[0048] The K prepared by 0.00741g embodiment 1 2 MnF 6 , 0.8197g NaAlO 2 , 0.1182g KHF 2 Dissolve in 10mL of hydrofluoric acid (49wt.%) solution, stir for 5 minutes to fully dissolve, then add 10mL of precipitant methanol dropwise at a rate of 0.2 mL / s, after all 10mL of methanol has been added dropwise, continue stirring at room temperature For 5 minutes, stop stirring to take out the precipitate, centrifuge, wash twice with acetone, and dry at 60°C in an air atmosphere to obtain the product K 2 NaAlF 6 :Mn 4+ Fluoride phosphor.
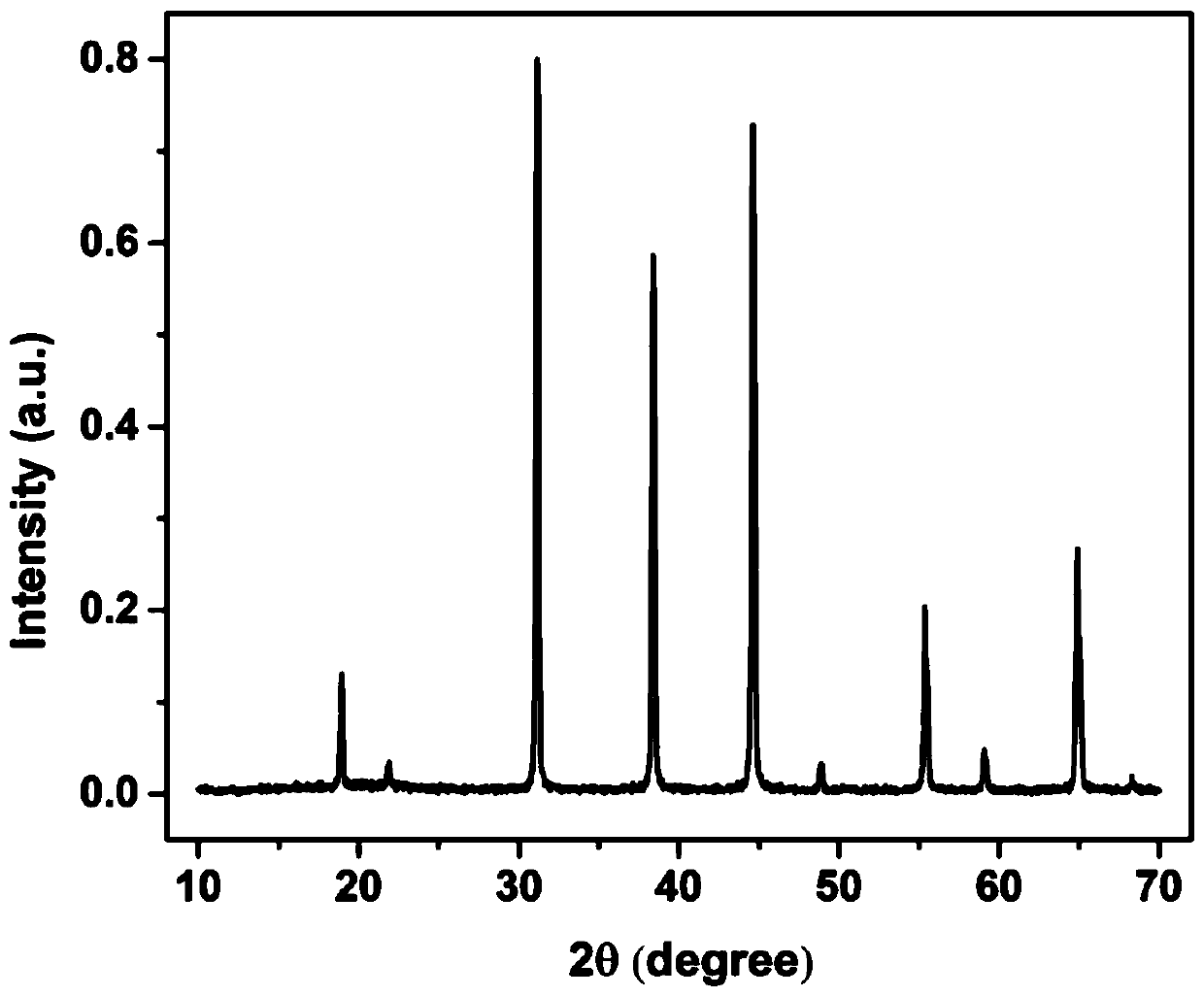
[0049] figure 1 for K 2 NaAlF 6 :Mn 4+ X-ray powder diffraction pattern of fluoride phosphor, indicating that the synthesized product is a square phase K 2 NaAlF 6 ; figure 2 for K 2 NaAlF 6 :Mn 4+ Scanning electron microscope image of fluoride phosphor powder, it can be seen that the particle size of the powder is about 1 μm; image 3 for K 2 NaAlF ...
Embodiment 3
[0051] Example 3: K 2 TiF 6 :Mn 4+ Preparation of Fluoride Phosphor Powder
[0052] The K prepared by 0.00741g embodiment 1 2 MnF 6 , 0.2401g K 2 TiF 6 Dissolve in 10mL of hydrofluoric acid (49wt.%) solution, stir for 5 minutes to fully dissolve, then add 10mL of precipitant methanol dropwise at a rate of 0.2mL / s, after all 10mL of methanol has been added dropwise, continue stirring at room temperature For 5 minutes, stop stirring to take out the precipitate, centrifuge, wash twice with acetone, and dry at 60°C in an air atmosphere to obtain the product K 2 TiF 6 :Mn 4+ Fluoride red phosphor.
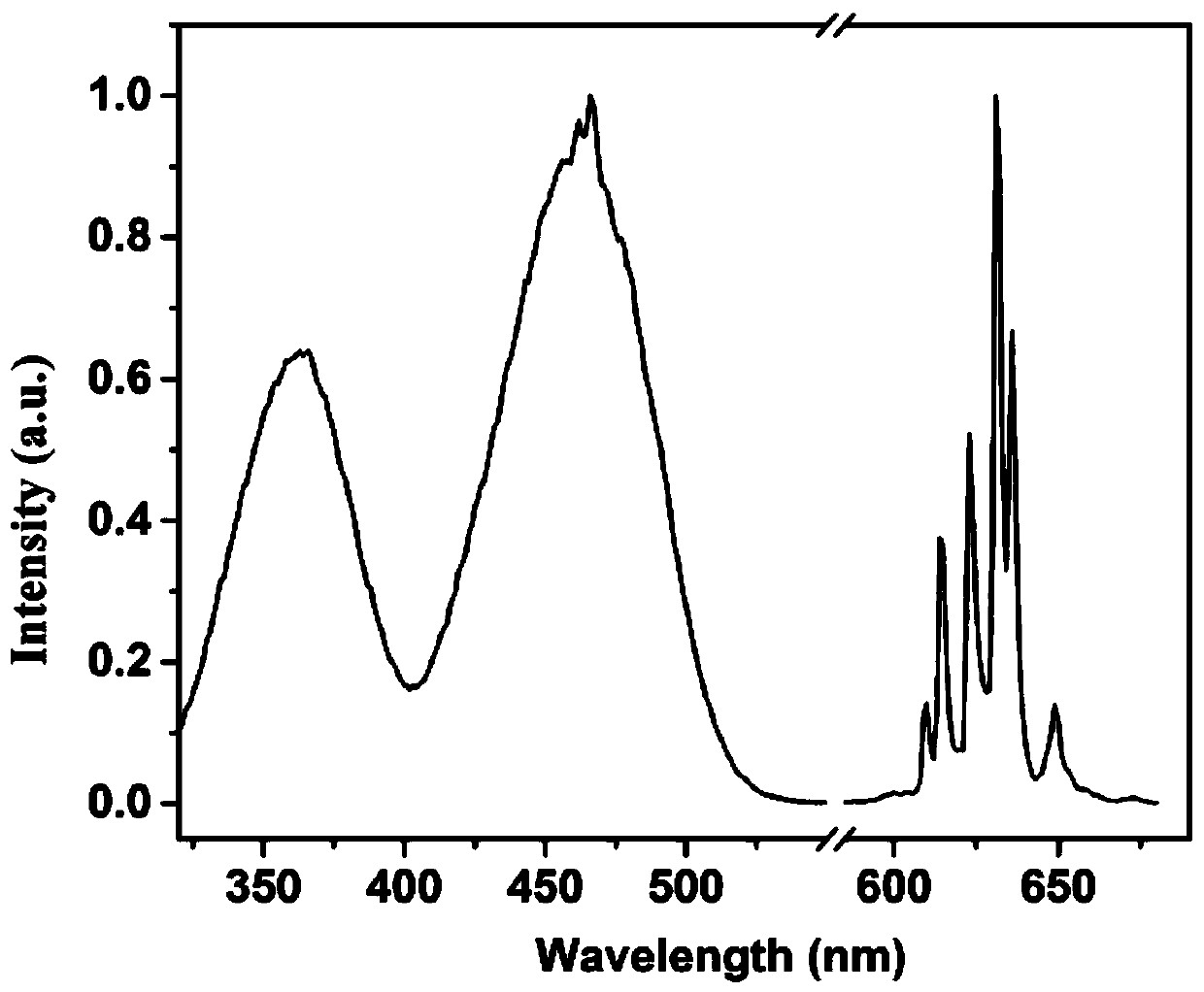
[0053] Figure 4 for K 2 TiF 6 :Mn 4+ X-ray powder diffraction pattern of fluoride phosphor, indicating that the synthesized product is a hexagonal phase K 2 TiF 6 ; Figure 5 for K 2 TiF 6 :Mn 4+ Scanning electron microscope image of fluoride phosphor powder, the powder particle size is about 10 μm; Figure 6 for K 2 TiF 6 :Mn 4+ Excitation and emission spectra o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com