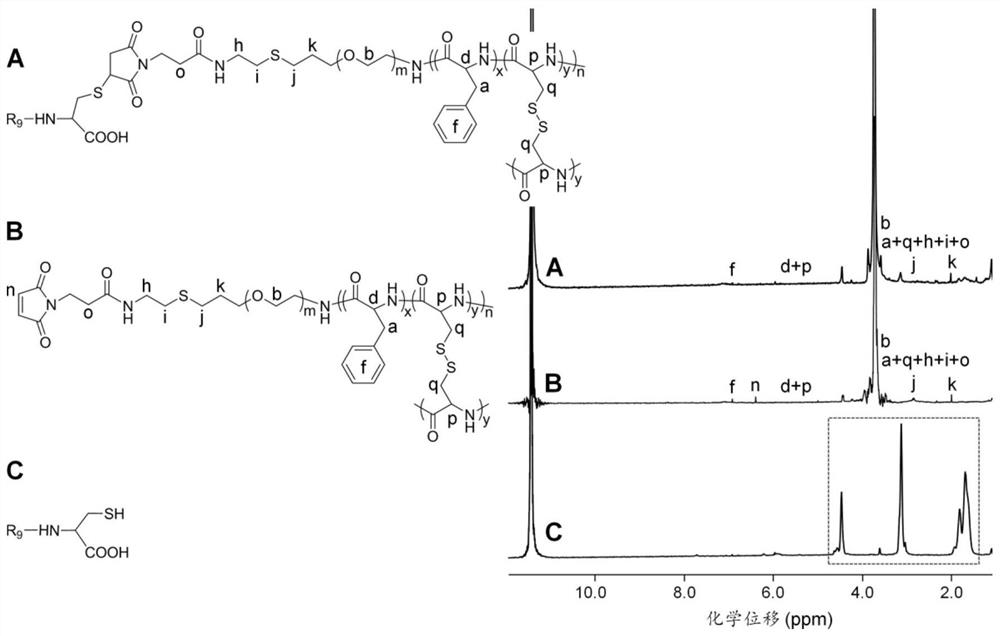
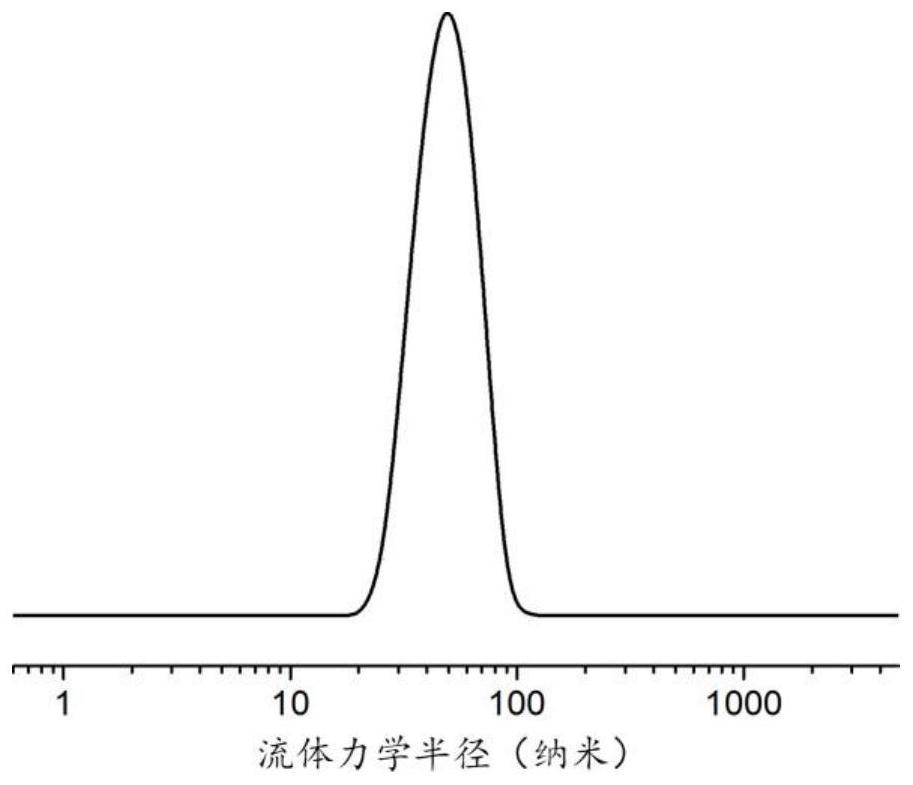
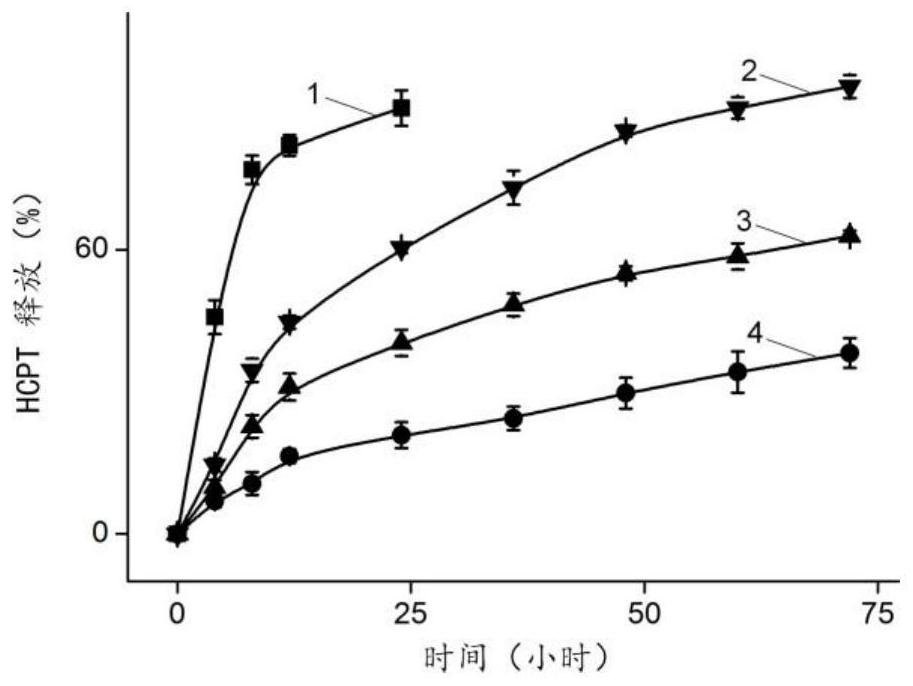
A nanogel, its preparation method and anti-tumor drug-loaded nanogel
A nano-gel and anti-tumor drug technology, applied in the field of polymer drug carriers, can solve the problems of high toxicity and side effects of drugs, poor water solubility and stability, and poor biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] The present invention also provides a method for preparing the nanogel described in the above technical solution, characterized in that, comprising the following steps:
[0088] a) Under the action of an initiator, react aminated allyl polyethylene glycol with 2-(Boc-amino)ethanethiol in the first organic solvent to form tert-butoxycarbonyl-polyethylene glycol-amino compound;
[0089] b) reacting the tert-butoxycarbonyl-polyethylene glycol-amino compound with L-cystine-N-cyclic anhydride and L-phenylalanine-N-cyclic anhydride in a second organic solvent, Formation of tert-butoxycarbonyl-imino-polyethylene glycol-poly(L-phenylalanine-co-L-cystine);
[0090] c) Under acidic conditions, the tert-butoxycarbonyl-imino-polyethylene glycol-poly(L-phenylalanine-co-L-cystine) is removed in a third organic solvent The reaction of tert-butoxycarbonyl forms the compound of formula (VII);
[0091]
[0092] d) dissolving the compound of formula (VII) in a fourth organic solvent...
Embodiment 1
[0191] Embodiment 1: Preparation of tert-butoxycarbonyl-polyethylene glycol-amino
[0192] Put 1g of aminated allyl polyethylene glycol in a dry reaction flask, add 10mL of N'N-dimethylformamide, then add 0.3g of 2-(Boc-amino)ethanethiol and 2.3g of azobis isobutyronitrile, stirred and reacted under nitrogen atmosphere for 3 days to obtain a reaction solution. The resulting reaction solution was poured into 100 mL of anhydrous ether, the solid was obtained by suction filtration, and dried in vacuo to obtain tert-butoxycarbonyl-polyethylene glycol-amino, namely the compound shown in formula (VI).
[0193]
[0194] Wherein, m is the degree of polymerization, 40≤m≤120.
Embodiment 2
[0195] Embodiment 2: the preparation of L-phenylalanine-N-anhydride in the ring
[0196] Mix 1g of L-phenylalanine and 0.6g of bis(trichloromethyl)carbonate at 25°C, add 50 mL of tetrahydrofuran, heat to 50°C for 2 hours, after the reaction, dissolve the reaction mixture in excess petroleum ether After sedimentation, separation, washing, recrystallization and drying, L-phenylalanine-N-cyclic acid anhydride is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com