Method for selectively depositing homogeneous nanoparticles on the surface of titanium dioxide microcrystals to enhance photocatalytic activity
A photocatalytic activity, titanium dioxide technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve problems such as low catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Measure 3mL of 30% hydrogen peroxide and 0.1mL of 35% hydrofluoric acid aqueous solution and uniformly disperse them in 27mL of deionized water, weigh 10mg of titanium powder and disperse them to form a reaction system;
[0027] 2) Transfer the above reaction system into a 50mL polymer-lined stainless steel hydrothermal kettle, and react at 180°C for 10 hours to obtain a precipitate;
[0028] 3) The prepared precipitate was washed three times with 6000r / min centrifugal water, then washed three times with 6000r / min centrifugal ethanol, dried at 60°C and then heat-treated at 400°C for 1 hour to prepare TiO with (001) surface exposed 2 Microcrystalline.
[0029] The field emission scanning electron microscope morphology of the sample prepared according to Example 1 is as follows: figure 1 shown. It can be seen from the figure that TiO 2 The microcrystal has a typical truncated octahedral morphology, consisting of two square (001) high-energy faces with top and bottom...
Embodiment 2
[0032] 1) Weigh 20 mg of TiO exposed on the (001) surface prepared according to Example 1 2 Microcrystalline, dispersed in 15 mL of TiF at a concentration of 0.04 mol / L 4 in aqueous solution;
[0033] 2) Transfer the solution into an oven at 60°C and let it stand for 6 hours;
[0034] 3) Separate and wash the precipitate.
[0035] The field emission scanning electron microscope morphology of the sample prepared according to Example 2 is as follows: image 3 shown. It can be seen from the figure that after reacting in the solution at 60°C for 6 hours, the TiO prepared according to Example 2 2 Uniform distribution of TiO on the (101) plane of the crystallite 2 Nanoparticles, with an average size of 50-80 nanometers. while successfully retaining TiO 2 The (001) high-energy facets with high photocatalytic activity in the microcrystals remain unchanged.
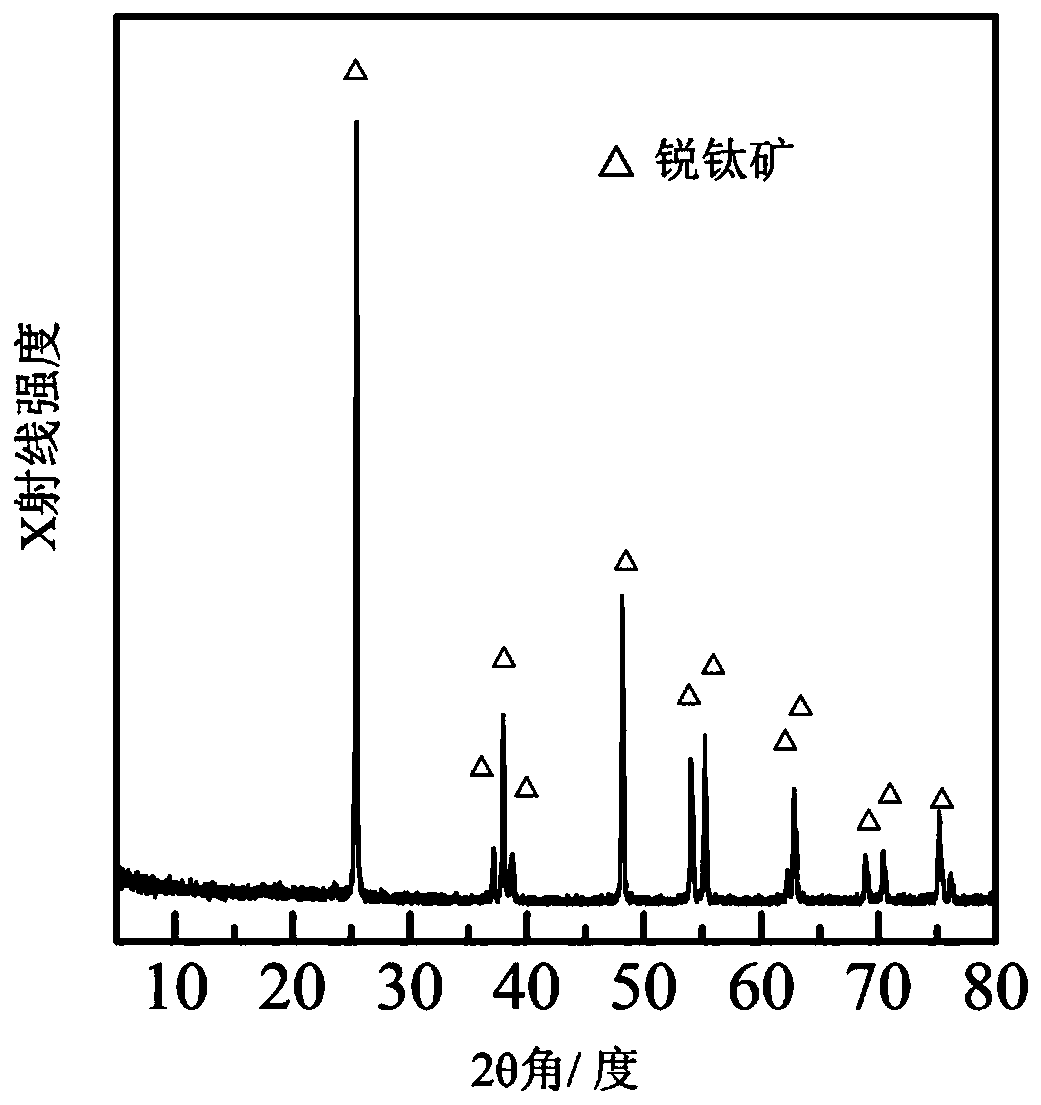
[0036] Prepare the X-ray diffraction pattern of sample according to embodiment 2 as Figure 4 As shown, according to JC...
Embodiment 3
[0038] 1) with embodiment 2;
[0039] 2) Transfer the solution to a 60°C oven and let it stand for 12 hours;
[0040] 3) Separate and wash the precipitate.
[0041] Figure 5 The microcrystalline morphology photographs show that the TiO 2 Selective deposition of nanoparticles on the (101) facets of the crystallites, leaving the high-energy (001) facets unaffected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com