Long wavelength photoinitiator containing carbazole derivatives and preparation method thereof
A technology of carbazole derivatives and photoinitiators, applied in the field of long-wavelength photoinitiators and their preparation, can solve problems such as toxicity and application limitations, and achieve the effects of enhanced conjugation, reduced dosage, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Weigh 2 mol of ethylcarbazole formaldehyde and 1 mol of tetrahydrothiopyrone dissolved in 20 ml of ethyl acetate, stir and mix well. Add 3-5 drops of 5% NaOH aqueous solution (0.5g NaOH, 9.5g water) to the prepared mixed solution dropwise, adjust the pH value to 13, and react under nitrogen protection at 30°C for 3h, then use an ice bath to continue the reaction 3h, pale yellow crystals were precipitated.
[0040] 2) Wash the light yellow crystal in step 1) with ethanol, dry in vacuo to remove the solvent, and obtain a pure light yellow product, the synthesized product is 2,6-(N-ethyl)-carbazoleethylene-tetrahydrothiopyrone .
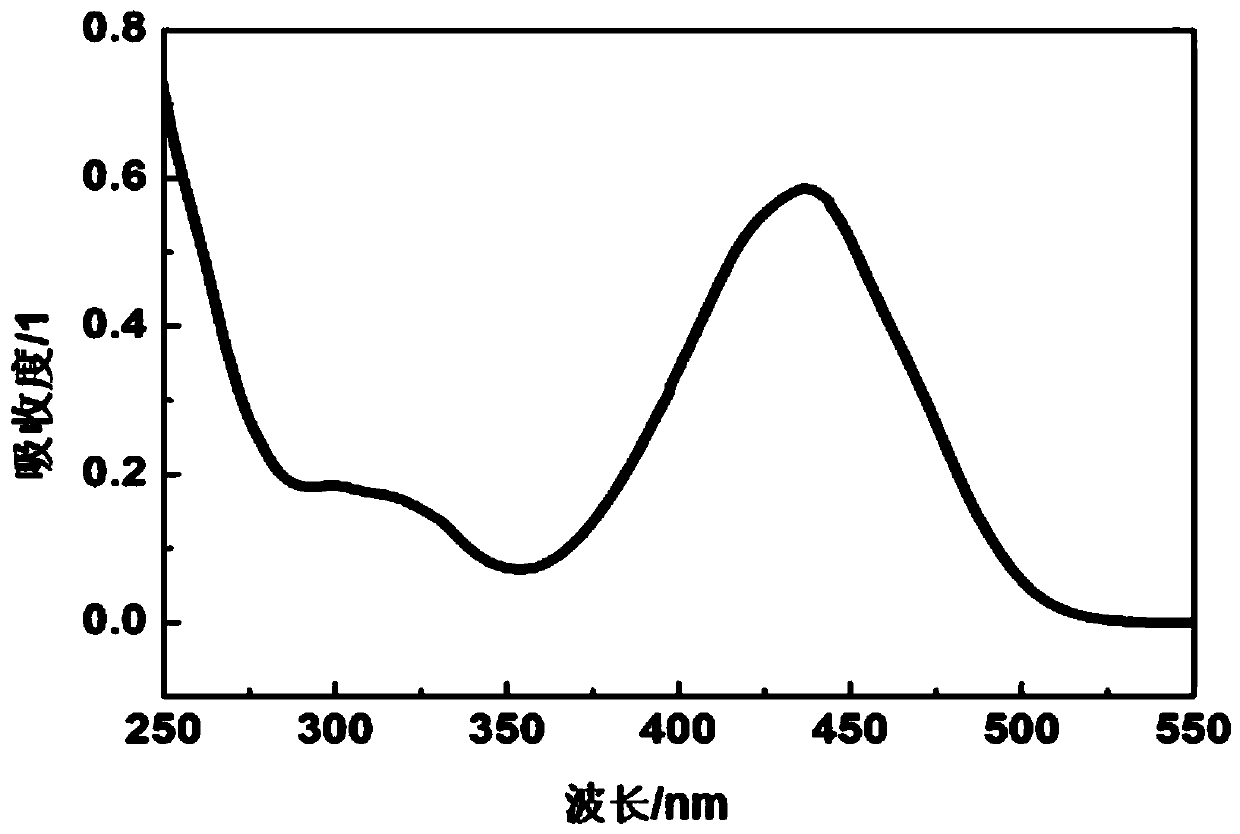
[0041] 2,6-(N-Ethyl)carbazoleethylene-tetrahydrothiopyrone at a molar concentration of 1×10 -5 Soluble in chromatographic grade acetonitrile, test its ultraviolet absorption spectrum, its maximum absorption wavelength can reach 500nm, such as figure 1 shown.
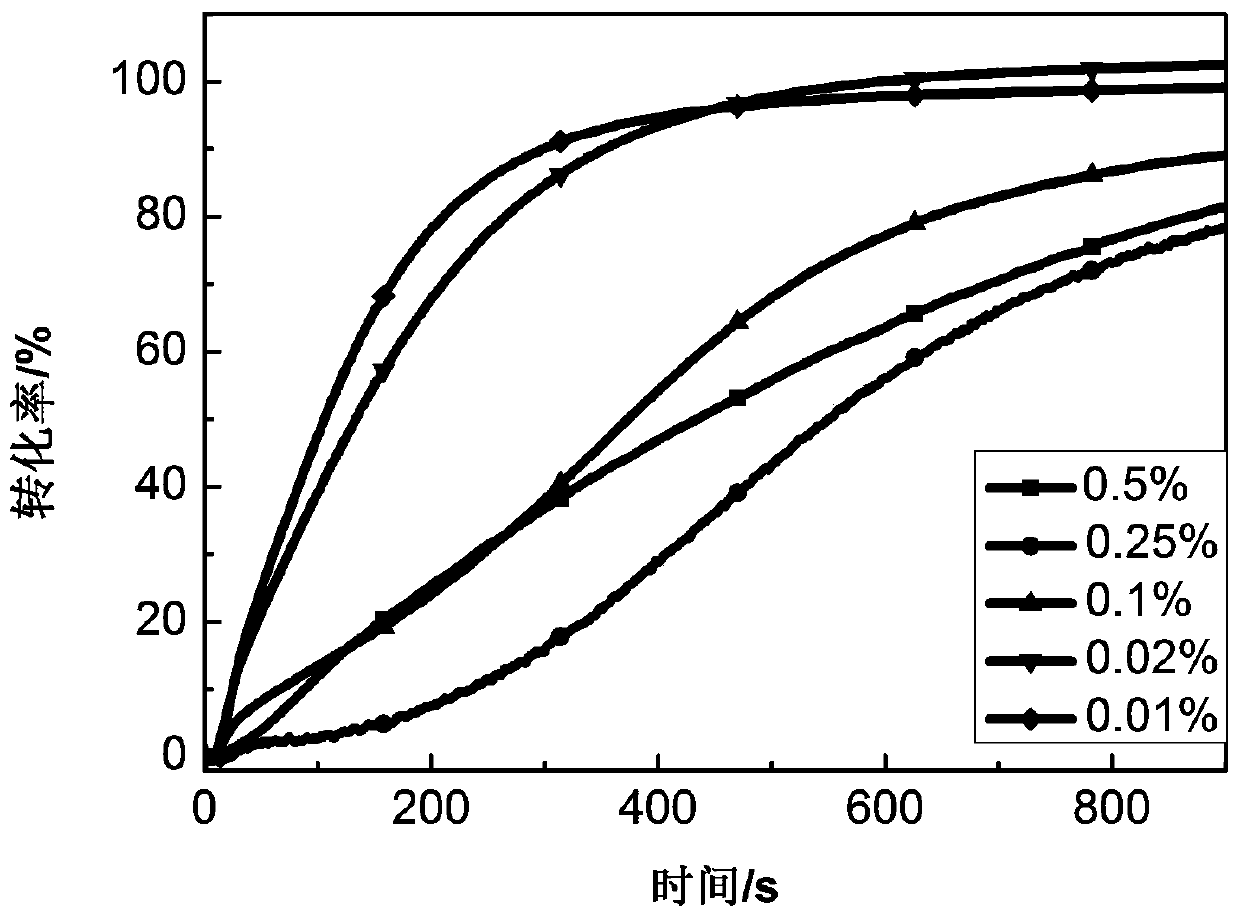
[0042] Dissolve 2,6-(N-ethyl)carbazoleethylene-tetrahydrothiopyrone in monomer P...
Embodiment 2
[0044] 1) Weigh 2 mol of ethylcarbazole formaldehyde and 1 mol of tetrahydropyrone and dissolve in 20 ml of ethyl acetate, stir and mix evenly, then add dropwise 3 to 5 drops of 5% NaOH solution (0.5 g NaOH, 9.5 g of water), adjust the pH to 13, react under nitrogen protection at 30° C. for 3 h, then use an ice bath to continue the reaction for 3 h, and pale yellow crystals are precipitated.
[0045] 2) The light yellow crystal in step 1) was washed with ethanol, and the solvent was removed by vacuum drying to obtain a pure light yellow product, which was 2,6-(N-ethyl)carbazoleethylene-tetrahydropyrone.
[0046] 2,6-(N-Ethyl)carbazoleethylene-tetrahydropyrone at a molar concentration of 1×10 -5 It is dissolved in chromatographic grade acetonitrile, and its ultraviolet absorption spectrum is tested. The results show that its maximum absorption wavelength can reach 500nm.
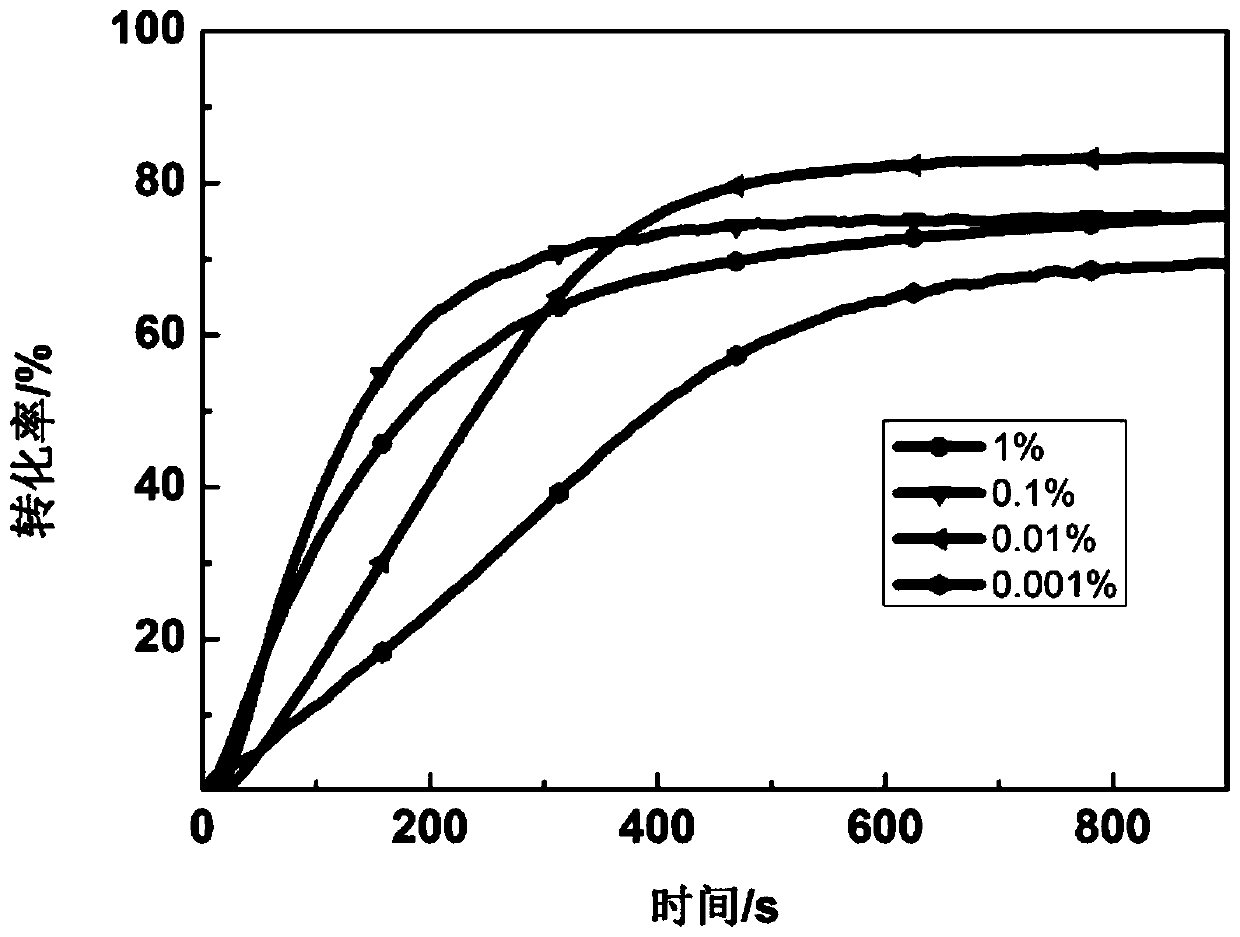
[0047] Dissolve 2,6-(N-ethyl)carbazoleethylene-tetrahydropyrone in PEGDA and HDDA at concentrations of 1%...
Embodiment 3
[0049]1) Weigh 2 mol of ethyl carbazole formaldehyde and 1 mol of tetrahydro-N-methylpiperidone and dissolve in 20 ml of ethyl acetate, stir and mix evenly. Add 3-5 drops of NaOH solution (0.5g NaOH, 9.5g water) with a mass fraction of 5% to the prepared mixed solution dropwise, adjust the pH value to 13, and react under nitrogen protection at 30°C for 3h, then use an ice bath to continue the reaction 3h, pale yellow crystals were precipitated.
[0050] 2) Wash the light yellow crystals in step 1) with ethanol, dry in vacuo to remove the solvent, and obtain a pure light yellow product, which is 2,6-(N-ethyl)carbazoleethylene-tetrahydro(N-methyl) base) piperidone.
[0051] Add 2,6-(N-ethyl)carbazoleethylene-tetrahydro(N-methyl)piperidone to a molar concentration of 1×10 -5 Soluble in chromatographic grade acetonitrile, test its ultraviolet absorption spectrum, its maximum absorption wavelength can reach 500nm.
[0052] 2,6-(N-ethyl)carbazoleethylene-tetrahydro(N-methyl)piper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com