3, 3-bipyridine complex and preparation method and application thereof
A technology for bipyridine and complexes, applied in the field of 3,3-bipyridine complexes and their preparation, can solve problems such as poor selectivity, low sensitivity, complex production process, etc., achieve high-yield production, simple raw materials, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The embodiment of the present invention also provides a preparation method of a 3,3-bipyridine complex, which is used for the above-mentioned 3,3-bipyridine complex, including:
[0023] After mixing p-2,5-furandicarboxylic acid, 3,3-bipyridine and zinc nitrate, they were added to N,N-dimethylacetamide:ethanol (volume ratio 1:1) solution and stirred for 10 minutes. Then place it in an oven at 95° C. for 72 hours, and obtain the above-mentioned 3,3-bipyridine complex after cooling.
[0024] In the above-mentioned method, the consumption of each raw material is:
[0025]
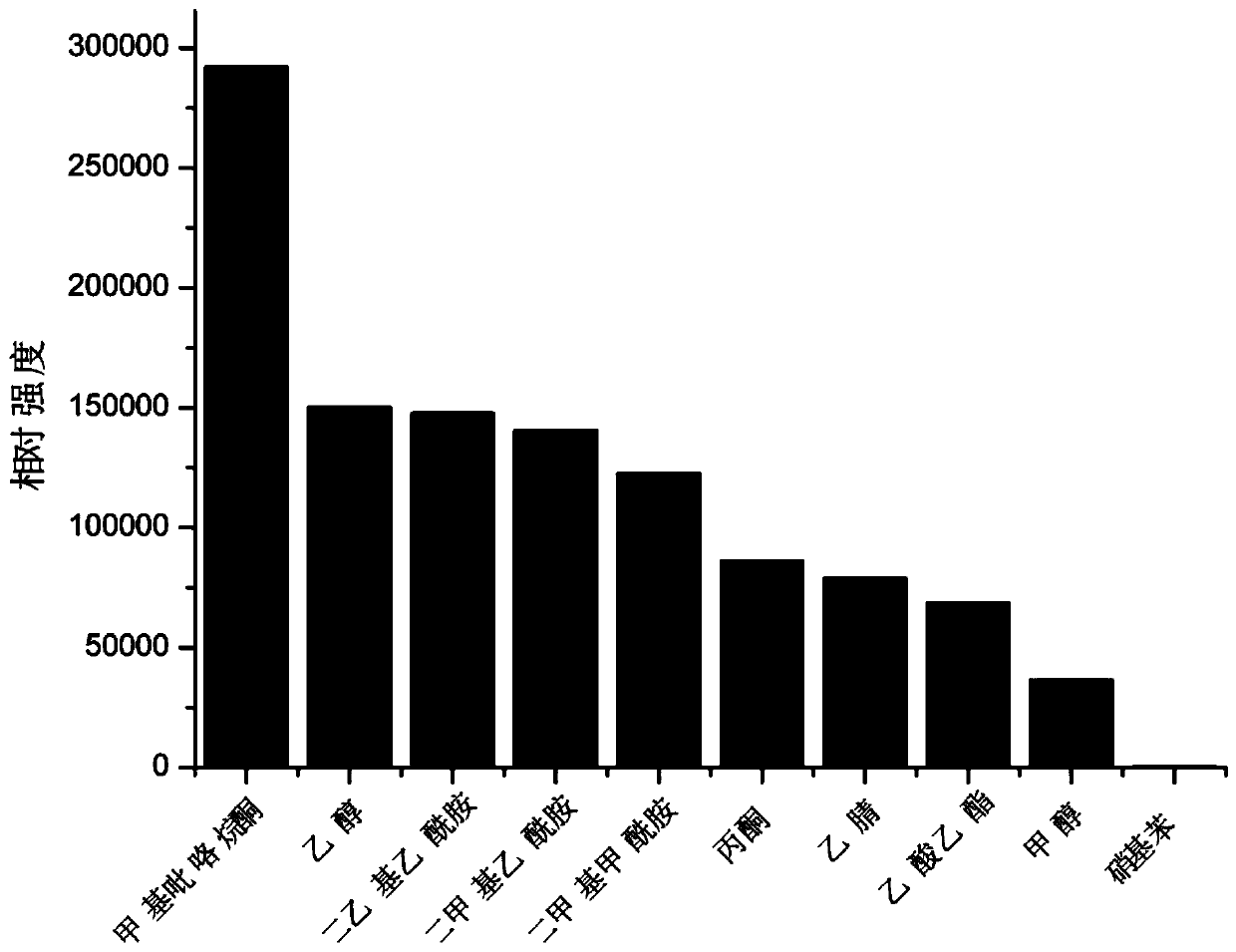
[0026] The embodiment of the present invention further provides an application of the above-mentioned 3,3-bipyridine complex as a fluorescent probe for recognizing nitrobenzene.
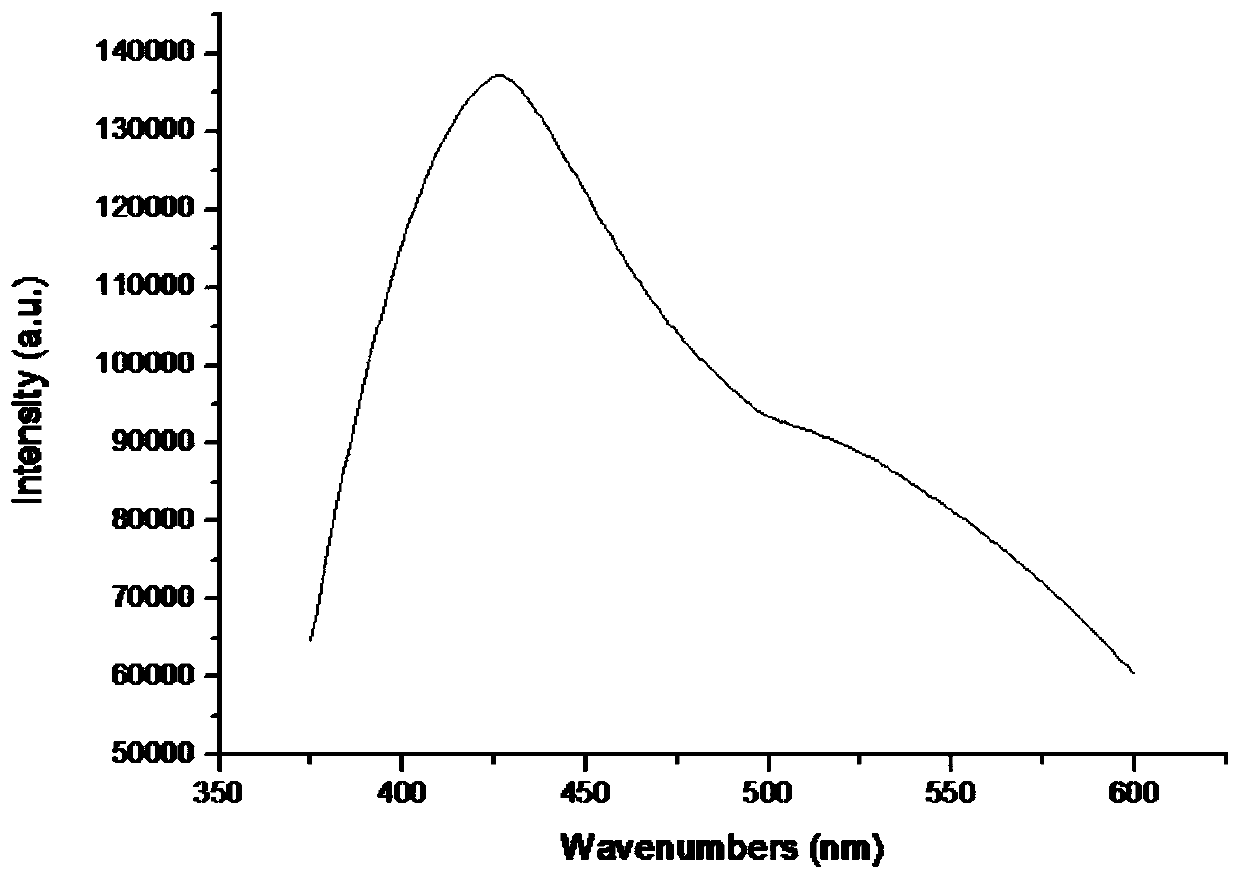
[0027] The 3,3-bipyridine complex of the invention has high sensitivity and selectivity to nitrobenzene, and can be used as a fluorescent probe for recognizing nitrobenzene with high sensitivity and high selectivity. The r...
Embodiment 1
[0030] This embodiment provides a 3,3-bipyridine complex, the preparation method of which comprises:
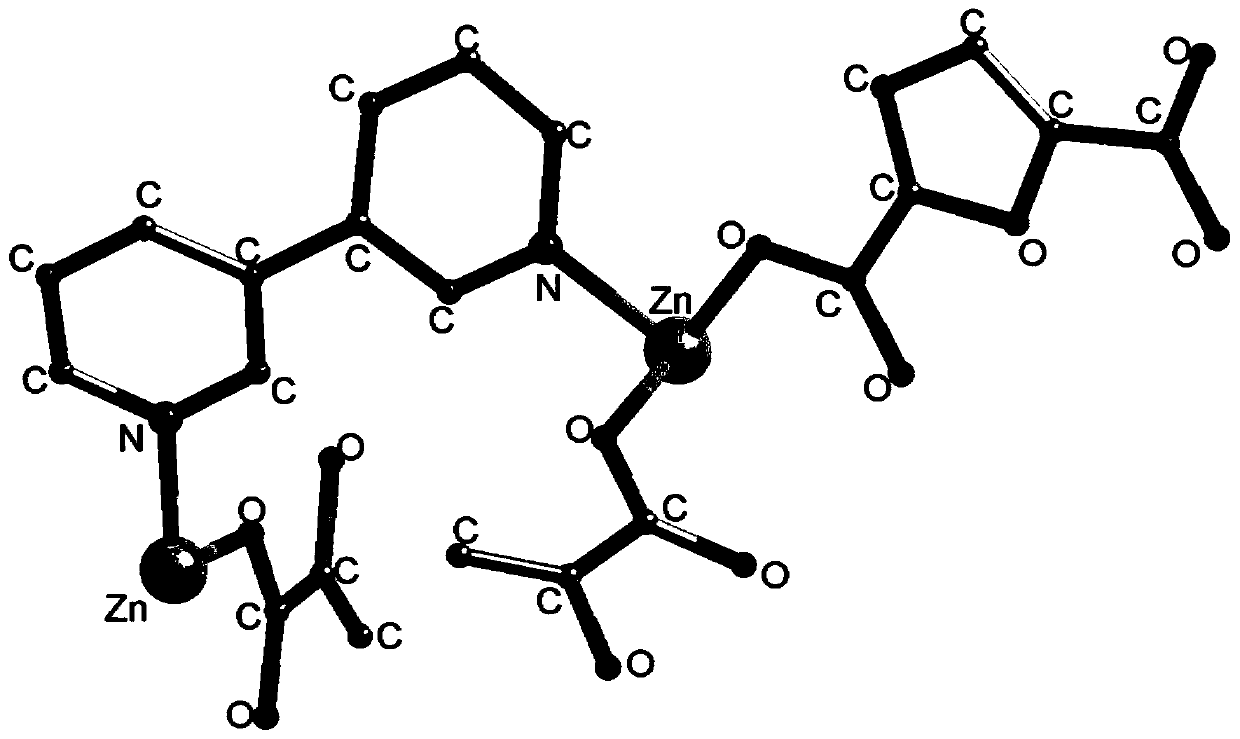
[0031] After mixing 0.016g of 2,5-furandicarboxylic acid, 0.016g of 3,3-bipyridine and 0.030g of zinc nitrate, add it to 5mL of N,N-dimethylacetamide and ethanol in a volume ratio of 1:1 The solution was stirred for 10 minutes, and then placed in an oven at 95°C for 72 hours. After cooling, the obtained colorless blocky crystals were 3,3-bipyridyl complexes.
[0032] Specifically, the selected size is 0.25×0.24×0.23mm 3 The single crystal structure analysis of the 3,3-bipyridine complex prepared in Example 1 of the present invention was carried out. The single crystal diffraction data was collected by a Bruker-AXS SMART APEX2 CCD diffractometer, and the Mokα monochromated by a graphite monochromator Rays 2.5°≤θ≤25.3°, thus the following results are obtained: the 3,3-bipyridine complex prepared in Example 1 of the present invention belongs to the monoclinic crystal system, ...
Embodiment 2
[0034] This embodiment provides a 3,3-bipyridine complex, the preparation method of which comprises:
[0035] After mixing 0.16g of 2,5-furandicarboxylic acid, 0.16g of 3,3-bipyridine and 0.30g of zinc nitrate, add it to 50mL of N,N-dimethylacetamide and ethanol in a volume ratio of 1:1 The solution was stirred for 10 minutes, and then placed in an oven at 95°C for 72 hours. After cooling, the obtained colorless blocky crystals were 3,3-bipyridyl complexes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Slit width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com