High-sensitivity fluorescent compound PPAB detecting organic primary amine with naked eyes and application of fluorescent chemical PPAB
A technology of fluorescent compounds and organic primary amines, which is applied in the field of analysis and detection, can solve the problems of low sensitivity and achieve the effects of improved sensitivity, rapid response and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
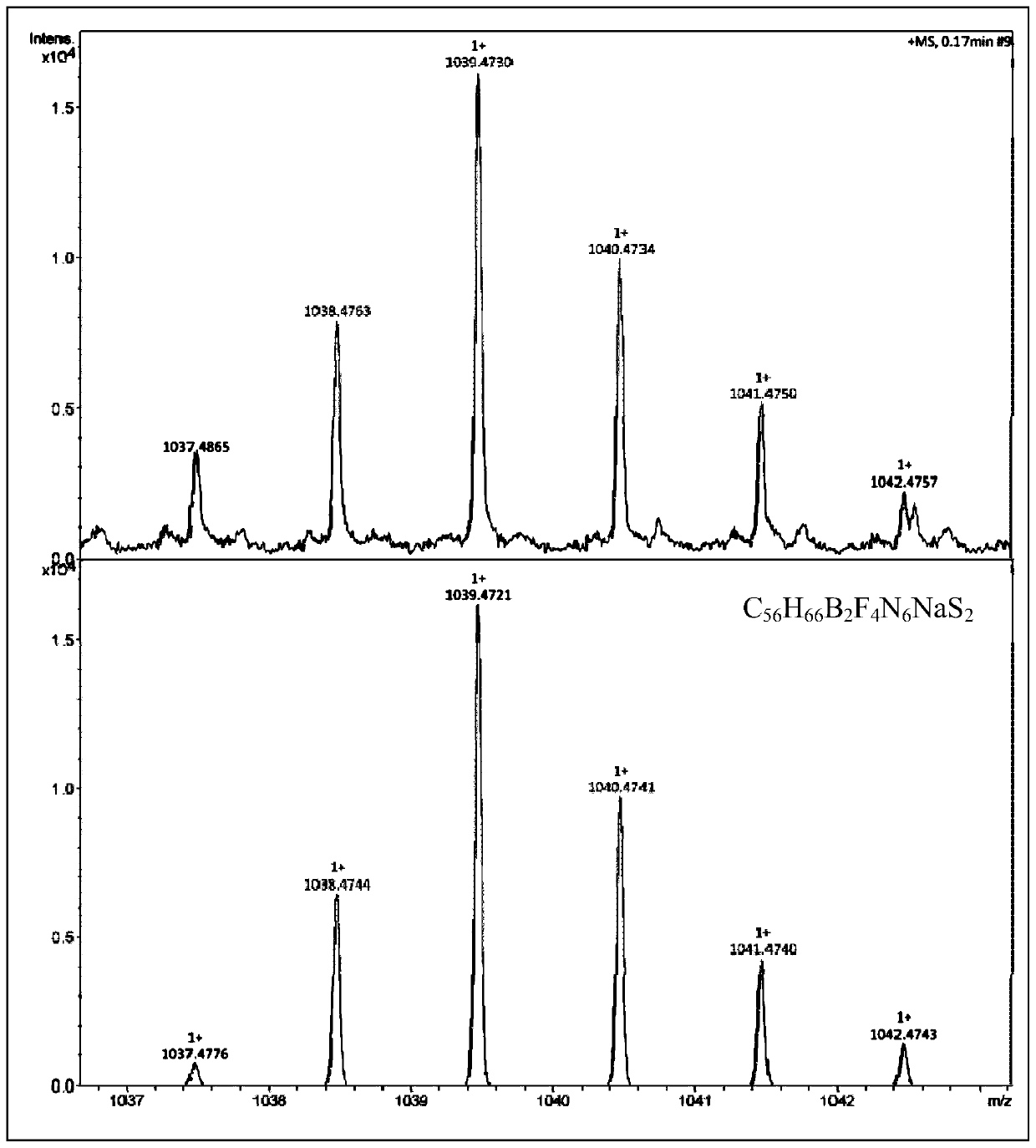
[0034] The reaction equation for preparing the fluorescent compound PPAB1 is:
[0035]
[0036] (1) Compound 1 was prepared according to the method disclosed in G.M.Fischer, M.Isomaeki-Krondahl, I.Goettker-Schnetmann, E.Daltrozzo and A.Zumbusch, Chemistry-A European Journal, 2009, 15, 4857-4864.
[0037] (2) Dissolve compound 1 (365mg, 0.67mmol) and compound 2 (1110mg, 5.36mmol) in 40mL toluene, heat the reaction system to 50°C, stir for 10 minutes, then add triethylamine (2.24g, 22.11mmol) and TiCl 4 (1.52g, 8.04mmol), TLC detects that after the raw material has reacted, add BF to the reaction solution 3 -Et 2 O (2.63g, 18.56mmol), continue to stir for 30 minutes, pour the reaction solution into 200mL water, and use CH 2 Cl 2 Extracted 3 times, anhydrous NaSO 4 Drying, filtration, rotary evaporation to remove the solvent, separation by silica gel column chromatography to obtain the crude product of PPAB1, which was washed with CH 2 Cl 2 Recrystallization from methan...
Embodiment 2
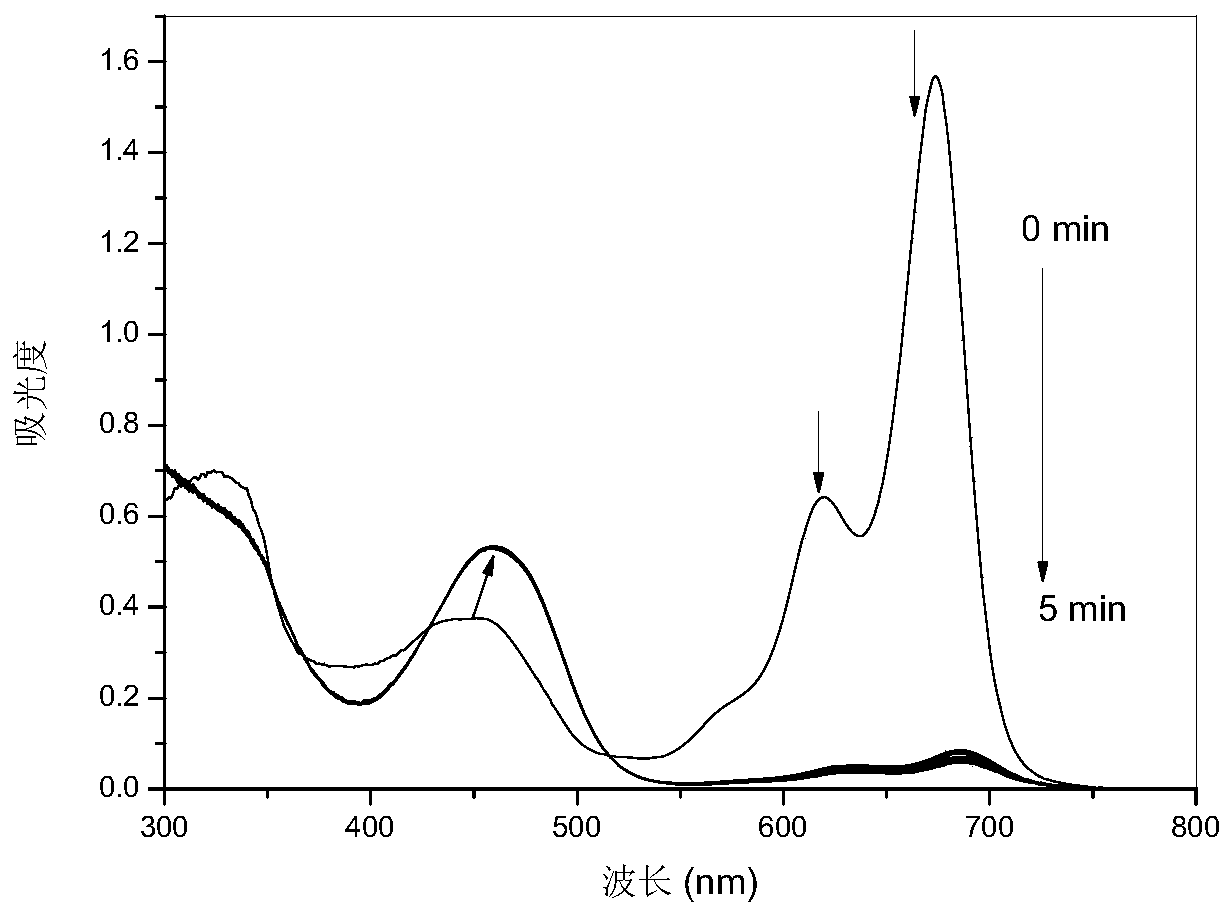
[0040] PPAB1 was dissolved in 1,4-dioxane to obtain the probe mother solution (10 -3 mol / L), followed by adding 1,4-dioxane to dilute to obtain 10 -5 mol / L PPAB1 solution, add 1,3-propanediamine solution (4×10 -5 mol / L), to test the change of its ultraviolet spectrogram in different reaction time, from figure 2 It can be seen that the intensity of the absorption peaks at 680nm and 630nm decreased rapidly, the absorption peak at 450nm shifted red to 480nm, and reached equilibrium in 3 minutes, and the color of the solution changed from green to yellow.
Embodiment 3
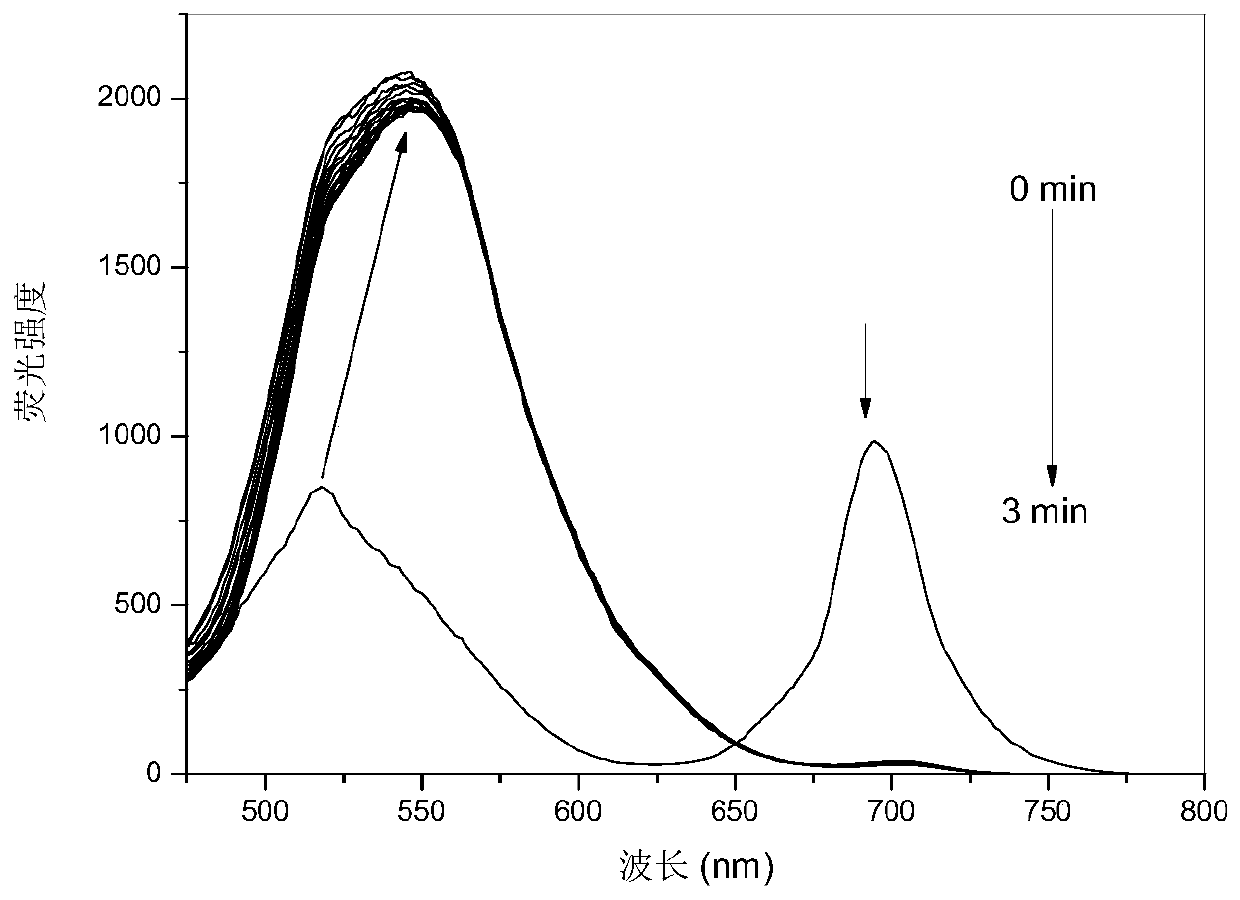
[0042] PPAB1 was dissolved in 1,4-dioxane to obtain the probe mother solution (10 -3 mol / L), followed by adding 1,4-dioxane to dilute to obtain 10 -5 mol / L PPAB1 solution, add 1,3-propanediamine solution (4×10 -5 mol / L), to test the change of its fluorescence spectrum in different reaction time, from image 3 It can be seen that the fluorescence intensity at 695nm decreased, the fluorescence at 518nm shifted to 544nm and reached equilibrium in 3 minutes with the increase of fluorescence intensity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com