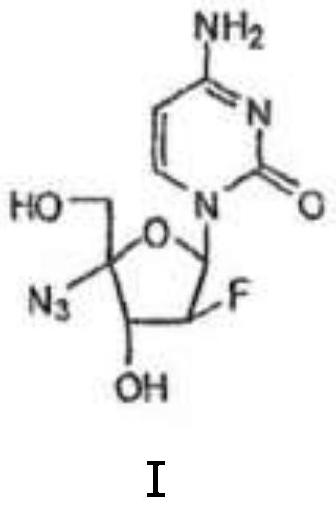
Crystal form a of 2'-fluoro-4'-substituted nucleoside analog i and its preparation method and application
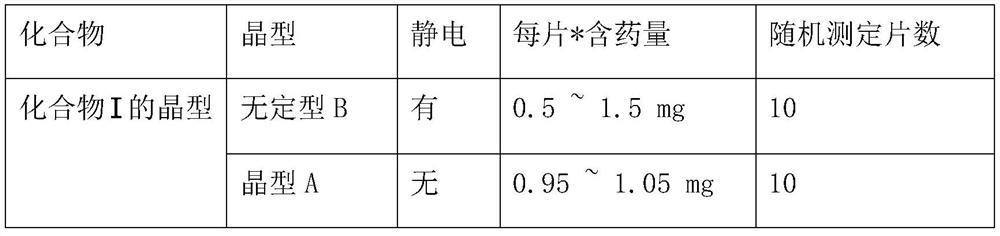
A technology of nucleoside analogs and crystal forms, which is applied in the field of its new crystal forms and its preparation, 2'-fluoro-4'-substituted nucleoside analogs I, to reduce drug resistance, no static electricity generation, uniformity Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
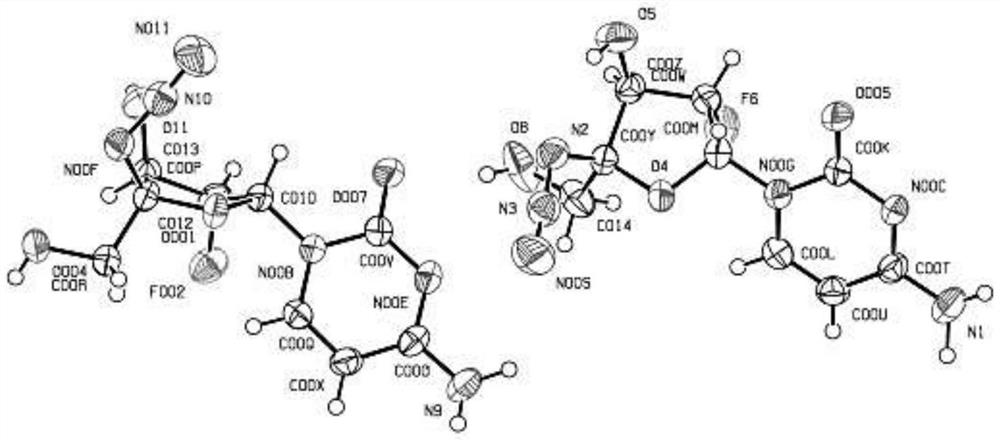
Image
Examples
Embodiment 1
[0029] Example 1. Preparation of Form B
[0030] 1.1 Suspension experiment
[0031] Dissolve the 2'-fluoro-4'-substituted nucleoside analog I of the present invention in one of the solvents selected below, and prepare the solution with a concentration of 7-20mg / mL. The solvent was evaporated with a rotary evaporator to obtain Form B.
[0032] The solvents used in this experiment are: methanol, ethanol, n-propanol, isopropanol, NN-dimethylformamide (DMF), ethyl acetate, isopropyl acetate, n-hexane, cyclohexane, water, ether , isopropyl ether, methyl tert-butyl ether, 4-methyl-dipentanone, tetrahydrofuran, acetonitrile, dichloromethane, chloroform.
[0033] 1.2 Rapid evaporation method experiment
[0034] Weighed 100 mg of compound I, added excess methanol solution and raised the temperature to dissolve, put it in a vacuum rotary evaporator at 50°C, and obtained a white solid as crystal form B after removing the solvent.
Embodiment 2
[0035] Example 2. Preparation of Form A
[0036] 2.1 Recrystallization experiment
[0037] Weigh a certain amount of 2'-fluoro-4'-substituted nucleoside analog I of the present invention, heat it to reflux with methanol, or ethanol, or n-propanol, or water to prepare its saturated solution, cool and crystallize to obtain crystal form A.
[0038] 2.2 Liquid surface diffusion experiment
[0039]Compound I was dissolved in a soluble solvent at 60°C, and the anti-solvent was slowly dripped into the sample solution along the wall, and cooled until the solid precipitated. The crystal form of the obtained crystal is Form A.
[0040] The easily soluble solvents used here are: methanol, DMF or water.
[0041] The anti-solvents used here are: n-hexane, cyclohexane, isopropyl ether or ethyl acetate
[0042] 2.3 Binary solvent experiments
[0043] Weigh 15 mg of compound I into a sample bottle, add 3 mL of binary mixed solvent, shake at 200 rpm at 50°C for 48 hours, filter, and slowl...
Embodiment 3
[0055] Embodiment 3. Antiviral effect of compound I crystal form A
[0056] The anti-HIV activity of compound I crystal form A was determined according to the literature method (Eur. J. Med. Chem. 2011, 46, 4178).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com