Fullerene phenylamine derivative as well as preparation method and application thereof
A technology of fullerene aniline and derivatives, which is applied in the field of fullerene aniline derivatives and their preparation, can solve problems such as poor stabilization effect and poor thermal stability of chemical stabilizers, and achieve novel structure, stable performance, and responsive The effect of simple and convenient conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Synthesis of hexachlorofullerene with fullerene and iodine monochloride
[0022] Add C to the 250mL flask 60 (0.48g, 0.68mmol) and 80mL of chlorobenzene, ultrasonically make C 60 completely dissolved. At room temperature (25°C), add ICl (1mL, 20.0mmol), C 60 :ICl molar ratio is about 1:30. Then replace the air atmosphere with N 2 system, in N 2 Reaction under atmosphere for 2h. Use a decompression device equipped with a cold trap to evaporate under reduced pressure until there is no solvent, and the process is controlled within 0.5h. Then an appropriate amount of CH 2 Cl 2 Dissolve the orange-red product in the bottle and spin dry to remove a small amount of iodine or iodine monochloride, repeating several times. It was then transferred to a 50 mL flask and spin-dried to obtain an orange-red solid product with a yield of 96%.
Embodiment 2
[0023] Embodiment 2: Preparation of fullerene aniline derivative F1
[0024]
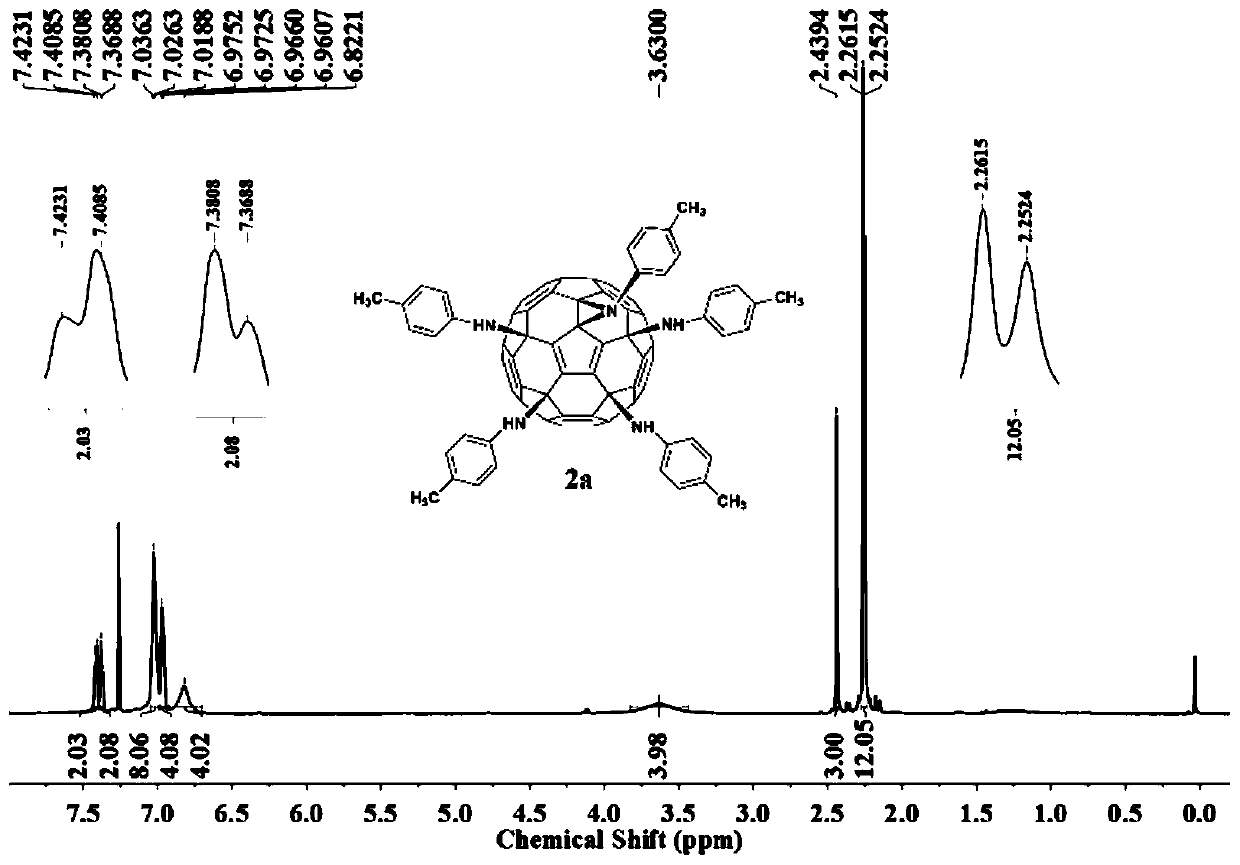
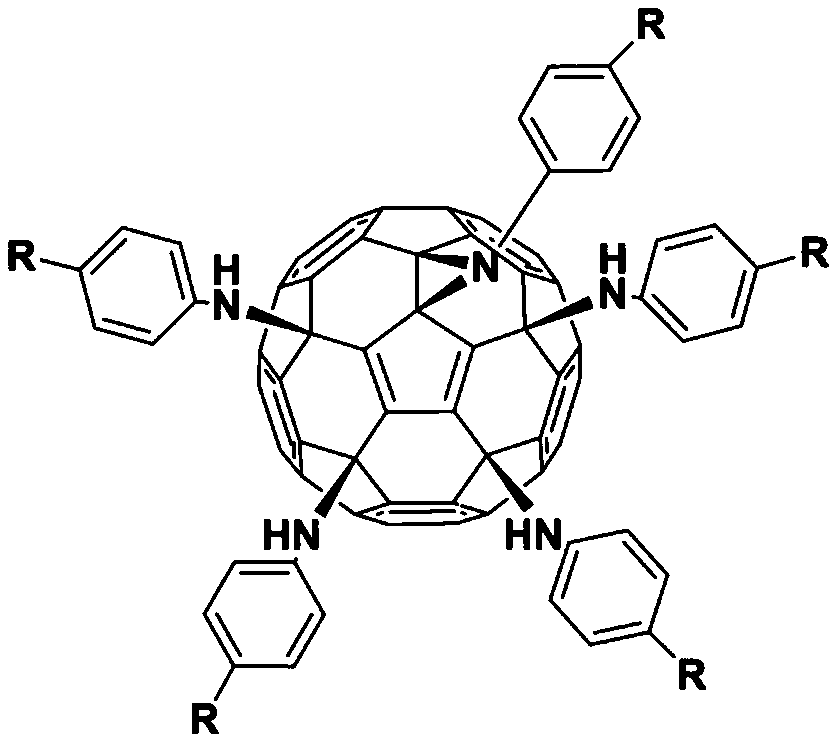
[0025] Weigh hexachlorofullerene (100mg, 0.11mmol) and dissolve it in 100ml toluene, sonicate to C 60 Cl 6 Completely dissolved, it was a transparent orange-red solution, an appropriate amount of aniline (1.1mmol, 100uL) was added, followed by triethylamine (150ul, 1.1mmol), stirred vigorously at room temperature, and reacted for 2h until TLC analysis showed C 60 Cl 6 basically disappeared. The organic phase was washed three times with water and then dried. Toluene was removed by evaporation under reduced pressure to obtain a crude product. Separation and purification by silica gel column chromatography, using toluene-ethyl acetate as the eluent, gradient elution to obtain an orange-red solution, which was spin-dried to obtain an orange-red solid product F1 with a yield of 45%. FT-IR (KBr pellet, v, cm -1 ):3400(N-H),3041(Ar-H),2921,2850,1636,1596,1490,1417(benzene ring),1384,1228(C-N),1196...
Embodiment 3
[0026] Embodiment 3: Preparation of fullerene methylaniline derivative F2
[0027]
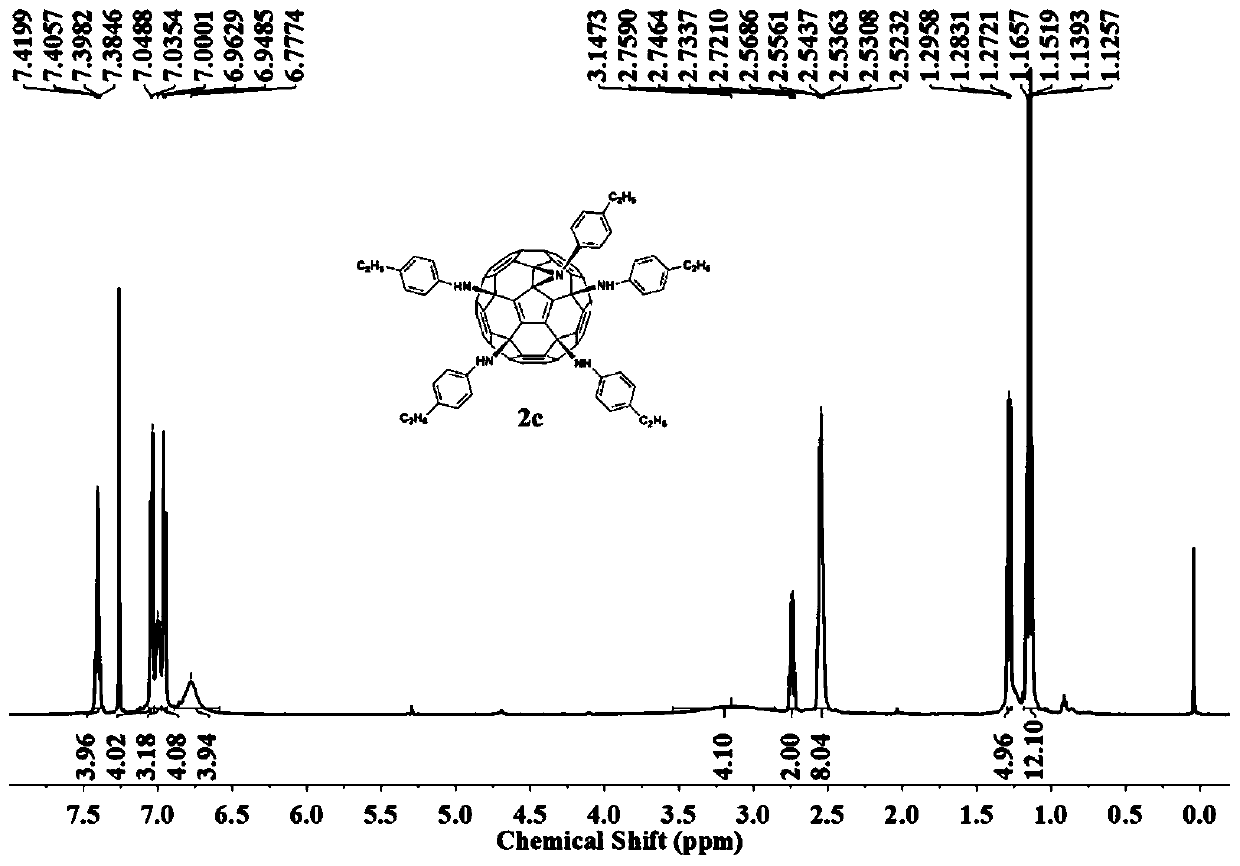
[0028] Weigh hexachlorofullerene (100mg, 0.11mmol) and dissolve in 80ml chlorobenzene, sonicate to C 60 Cl 6 Completely dissolved, it was a transparent orange-red solution, an appropriate amount of methylaniline (1.1mmol, 118mg) was added, followed by triethylamine (150ul, 1.1mmol), stirred vigorously at room temperature, and reacted for 2h until TLC analysis showed C 60 Cl 6 basically disappeared. The organic phase was washed three times with water and then dried. Chlorobenzene was removed by evaporation under reduced pressure to obtain a crude product. Separation and purification by silica gel column chromatography, using toluene-ethyl acetate as the eluent, gradient elution to obtain an orange-red solution, which was spin-dried to obtain an orange-red solid product F2 with a yield of 58%. FT-IR (KBr pellet, ν, cm-1 ):3400(N-H),2918,2852(C-H),1608,1508,1458(benzene ring),1230(C-N),11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com