Kit for detecting drug concentration of nilotinib in dried blood spots, and detection method implemented by kit
A technology of nilotinib and drug concentration, applied in the field of kits for the detection of nilotinib drug concentration, can solve the problems of time-consuming and laborious development, affecting accuracy, large sampling volume, etc. Inaccurate sampling, the effect of ensuring accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: kit composition
[0046] A kit for detecting the drug concentration of nilotinib in dried blood spots, comprising: an internal standard extraction reagent, a dried blood spot calibration product and a dried blood spot quality control product. in,
[0047] The internal standard extraction reagent uses D6-nilotinib as the internal standard, and the solvent of the internal standard extraction reagent is a mixture of organic solvent and water; the organic solvent is methanol, or acetonitrile, or a mixture of methanol and acetonitrile, and the organic solvent and water The volume ratio is (1.5~4.0):1.
[0048] The nilotinib dried blood spot calibration product is a group of dried blood spot samples of nilotinib with different concentration gradients, the number of the dried blood spot samples is at least 6, and the dried blood spot samples are fixed on the blood spot Acquisition card; the concentration range of the concentration gradient is 50-4000ng / mL. Suc...
Embodiment 2
[0076] Embodiment 2: Nilotinib DBS spotting volume to the research of detection result
[0077] The LC-MS / MS method was used to detect the concentration of nilotinib in dried blood slices, and the influence of sample volume on the detection results was studied.
[0078] Whole blood with an HCT value of 40% was used to prepare a high-concentration sample (high) and a low-concentration sample (low), and the sample concentration was the same as that of HQC and LQC.
[0079] Preparation of volume effect blood spot card: Take the above HQC and LQC samples, and put 10uL, 15uL, 20uL, 40uL on the DBS card respectively. Each sample was repeated three times and dried at room temperature for later use.
[0080] Experimental results:
[0081]
[0082] Acceptable standard: Investigate the detection concentration when the sample volume is 10μL, 15μL, 20μL, 30ul or 40μL. When the CV≤15%, the deviation from the theoretical value is ≤±20%, it is considered that the volume effect has no i...
Embodiment 3
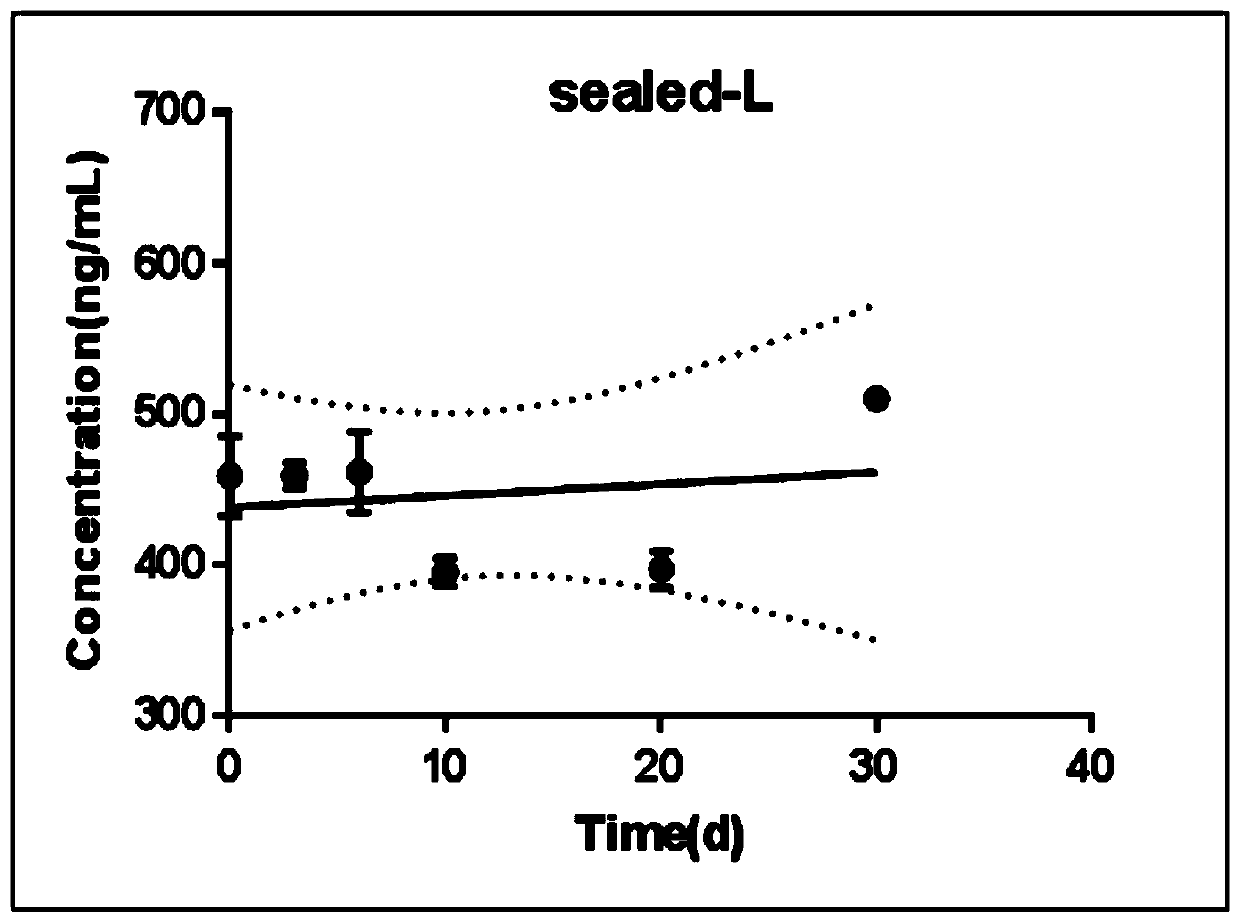
[0085] Embodiment 3: Nilotinib DBS accelerated stability test
[0086] Using the preparation method and detection method of the kit described in the above examples, the samples are preserved in an environment more severe than daily operation (high temperature 40°C and high humidity 75%), and the storage stability of the samples is tested in a shorter time.
[0087] Statistics of experimental results: refer to the statistical method of long-term stability of EP25-A, take time as the abscissa, and the average concentration detected at each time point as the ordinate to make a scatter plot, and use linear regression analysis to obtain the regression equation Y=bX+a .
[0088] 1) Make a t-test between the slope b and 0, if there is no significant difference (P>0.05), the sample remains stable during the detection period.
[0089] 2) If the slope b is significantly different from 0 (P<0.05), then draw the 95% one-sided confidence line of the regression line, which is parallel to X...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com