Glucopyranose-substituted pyrazole compound and preparation method thereof
A compound and reaction technology, applied in the field of glucopyranose-substituted pyrazole compounds and its preparation, can solve the problems of low purity of the final compound and unfavorable drug preparation process, so as to ensure controllable drug quality, easy industrial production, The effect of easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
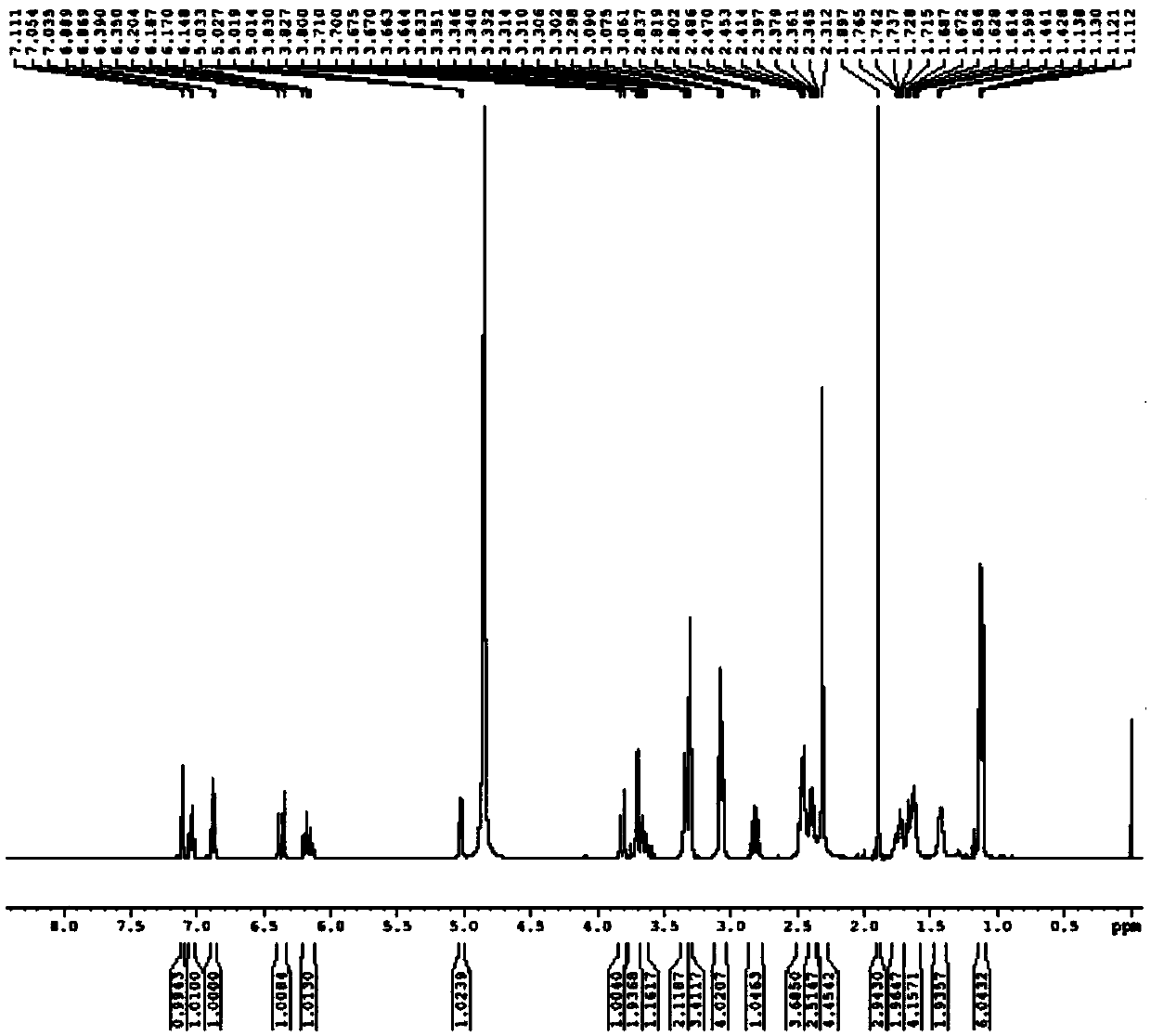
Image
Examples
Embodiment 1
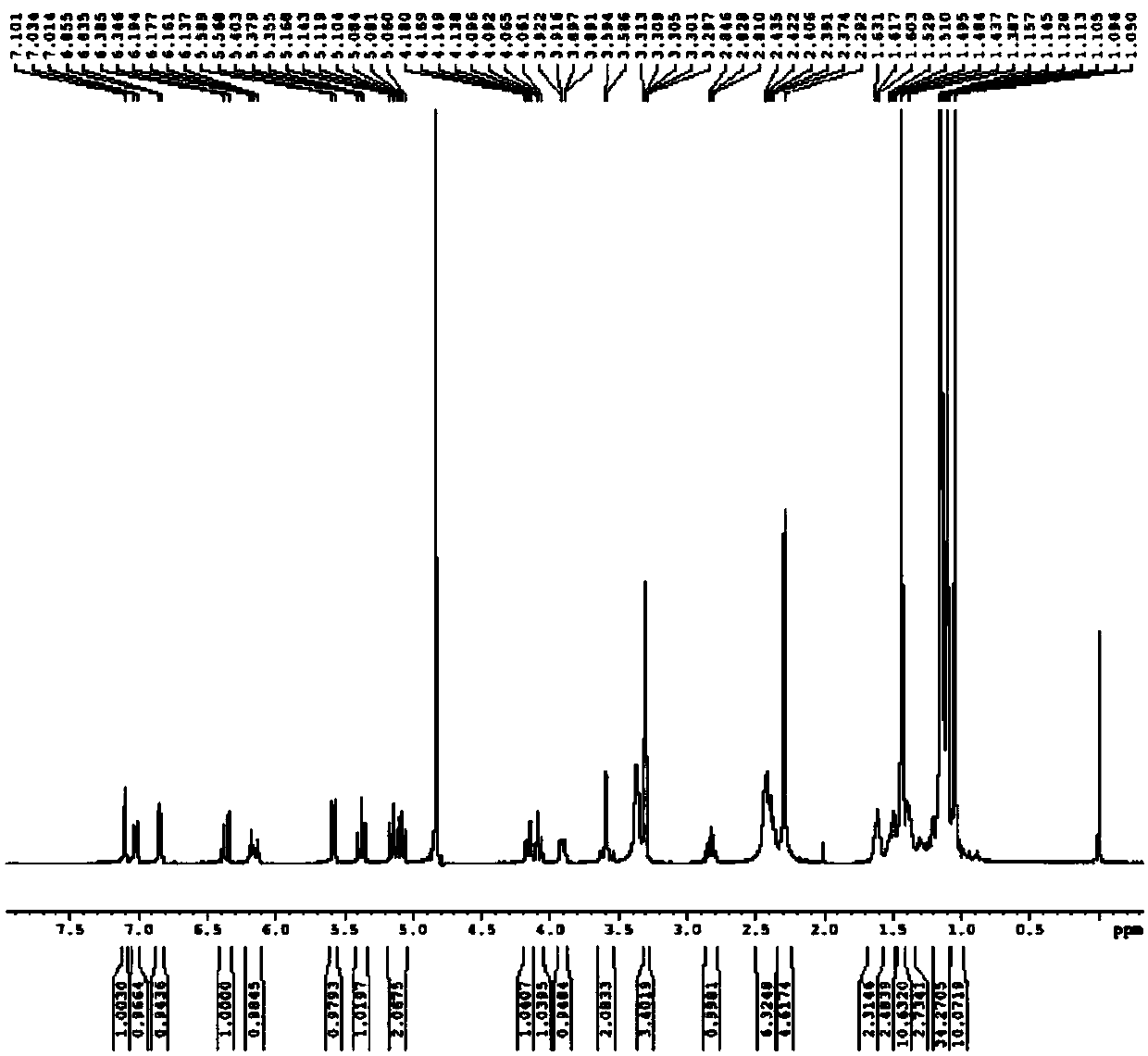
[0072] 626g of compound (16), 6L of acetonitrile, 840g of cesium carbonate and 1770g of 2,3,4,6-tetra-O-pivaloyl-α-D-glucosyl bromide (compound (17)) were successively added to the reactor, Heat to 40°C-45°C, react for 4-5 hours, then cool down to 20-25°C, filter, rinse the solid with acetonitrile once; dissolve the filter cake with 8L ethyl acetate and 10L water and separate the liquid, and concentrate the organic phase to about 3L , add 10L of acetonitrile, stir for 12h, precipitate out solid, filter, rinse the filter cake with acetonitrile, and dry in vacuum at 60°C for 24h to obtain 652g of off-white solid compound (9c), yield 61%, HPLC purity 98.52%. 1HNMR (400MHz, MeOD) ( figure 1 ): δ7.10(s, 1H), 7.03(d, J=8.0Hz, 1H), 6.86(d, J=8.0Hz, 1H), 6.39(d, J=15.6, 1H), 6.19-6.12( m, 1H), 5.59(d, J=8.4Hz, 1H), 5.40-5.35(t, J=9.6Hz, 1H), 5.17-5.06(m, 2H), 4.18-4.14(dd, J=12.4Hz , 4.4Hz, 1H), 4.10-4.06(dd, J=12.4Hz, 1.6Hz, 1H), 3.92-3.89(dd, J=10Hz, 2.4Hz, 1H), 3.64-3.54(dd, J=20...
Embodiment 2
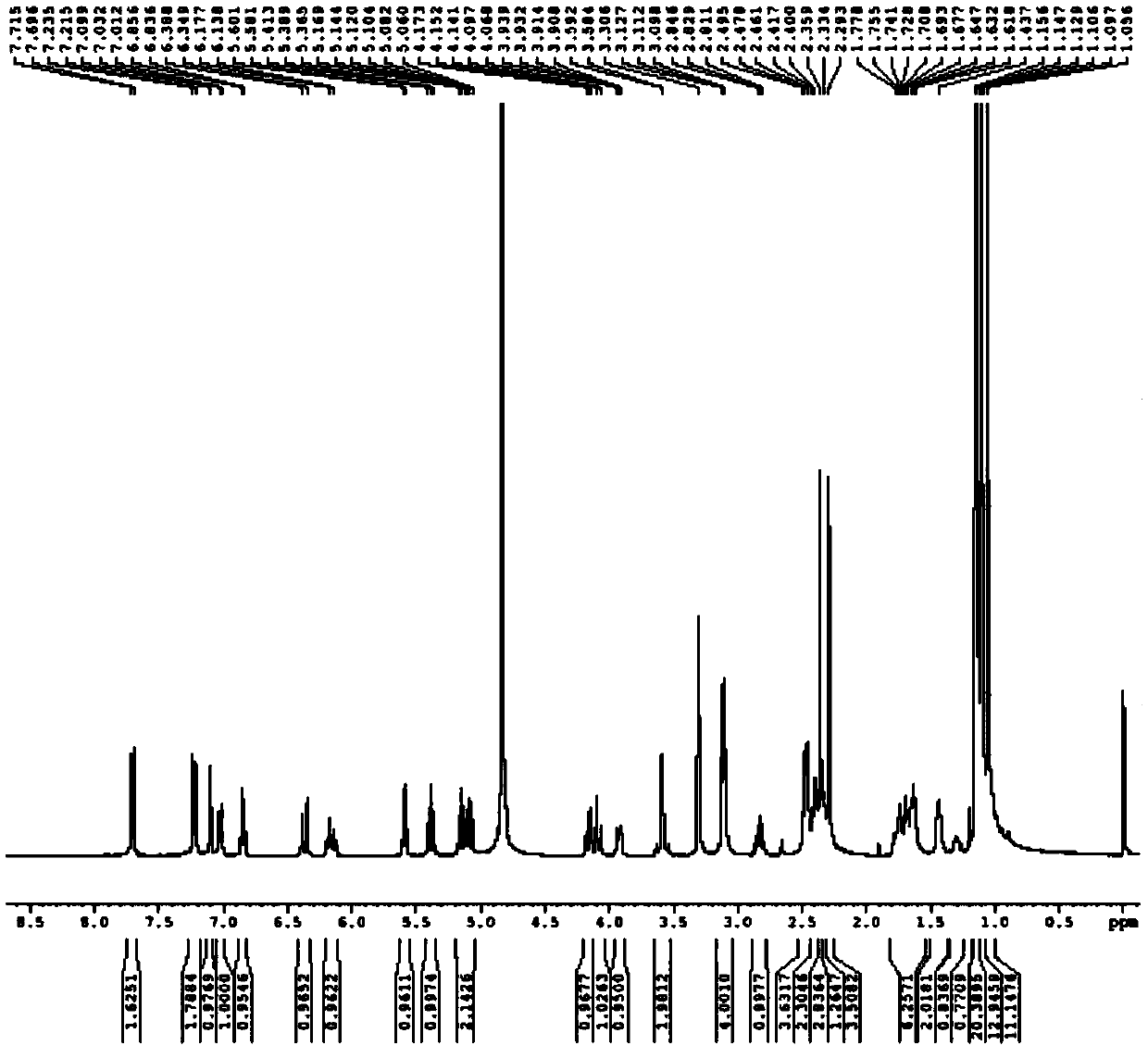
[0076] With the maleate of 5.00kg compound (16), 40L tetrahydrofuran, 5.47kg potassium phosphate and 11.67kg2,3,4,6-tetra-O-pivaloyl-α-D-glucosyl bromide (compound (17) ) into the reaction kettle in turn, heated to 40-45°C, cooled to 15-25°C after reacting for 4-5 hours, filtered, and rinsed the solid with tetrahydrofuran once. The filter cake was dissolved with 36L of ethyl acetate and 20L of water and separated. The organic phase was concentrated to about 18L, 64L of acetonitrile was added, and stirred for 15h. After filtering, the filter cake was rinsed with acetonitrile, and dried under vacuum at 60°C for 24 hours to obtain 4.50 kg of off-white solid compound (9c), with a yield of 57% and a purity of 99.19% by HPLC.
[0077] 4.45kg of compound (9c) and 45L of butyl acetate were added to the reaction kettle in turn, and the temperature was lowered to 15°C-20°C. Add 4.13kg of methanesulfonic acid in batches and react for 2-3 hours. Add 22L of 9% aqueous potassium hydroxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com