Carbon-resistant and sulfur poisoning-resistant solid-state oxide fuel cell positive electrode and preparation method thereof
A solid oxide and fuel cell technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of cumbersome and repetitive impregnation methods, poor catalytic activity, and low electrocatalytic activity, and achieve excellent anti-carbon deposition and anti-sulfur poisoning , good structural stability, high electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
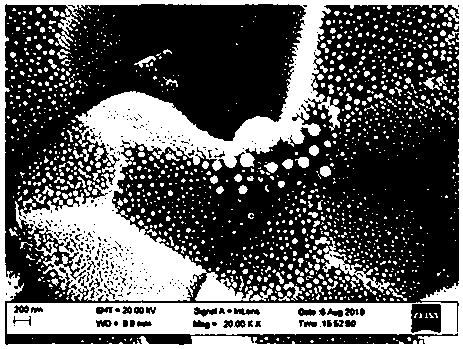
[0026] Cerium nitrate, samarium nitrate and copper nitrate are dissolved in deionized water to form a 1.0M nitrate solution, wherein the molar ratio of cerium ions to samarium ions is 4:1, and the molar content of copper ions accounting for all metal ions is 10%. Glycine is then added to the nitrate solution, wherein the molar ratio of glycine to nitrate in the solution is 1:2. Stir well at room temperature, heat on an electric furnace, concentrate until spontaneous combustion to obtain a light brown powder. Then heat treatment at 700 ° C for 4 h to obtain light brown CSCO (copper samarium co-doped cerium oxide) powder, the scanning electron microscope picture of the powder section is figure 1 shown.
[0027] The obtained CSCO powder was ball-milled for 72 hours, and the ball-milling medium was alcohol, and then vacuum-dried at 60° C. for 24 hours. The ball-milled powder and polystyrene monodisperse microspheres with a particle size of 0.3 μm were ultrasonically mixed in an ...
Embodiment 2
[0031] Dissolve cerium nitrate, gadolinium nitrate and copper nitrate in deionized water to form a 1.0M nitrate solution, wherein the molar ratio of cerium ions to gadolinium ions is 4:1, and the molar content of copper ions in all metal ions is 10%. Glycine is then added to the nitrate solution, wherein the molar ratio of glycine to nitrate in the solution is 1:2. Stir well at room temperature, heat on an electric furnace, concentrate until spontaneous combustion to obtain a light brown powder. Then heat treatment at 800°C for 5h to obtain light brown CGCO (copper-gadolinium co-doped cerium oxide) powder.
[0032] The prepared CGCO powder was ball-milled for 72 hours, and the ball-milling medium was alcohol, and then vacuum-dried at 50°C for 48 hours. The vacuum-dried powder and ethyl cellulose were ultrasonically mixed in alcohol medium at a mass ratio of 9:1 for 6 hours, and then vacuum-dried at 50°C for 48 hours. Put 0.6g of the obtained powder into a stainless steel mold ...
Embodiment 3
[0035] Cerium nitrate, samarium nitrate and copper nitrate are dissolved in deionized water to form a 0.5M nitrate solution, wherein the molar ratio of cerium ions to samarium ions is 4:1, and the molar content of copper ions accounting for all metal ions is 5%. Then stearic acid is added to the nitrate solution, wherein the molar ratio of stearic acid to nitrate in the solution is 2:1. Stir well at room temperature, heat on an electric furnace, concentrate until spontaneous combustion to obtain a light brown powder. Then heat treatment at 500 °C for 8 h to obtain light brown CSCO powder.
[0036] The obtained CSCO powder was ball-milled for 96 hours, the ball-milling medium was acetone, and then vacuum-dried at 70° C. for 12 hours. The milled powder and polystyrene monodisperse microspheres with a particle size of 0.3 μm were ultrasonically mixed in an acetone medium at a mass ratio of 6:1 for 1 h, and then vacuum-dried at 70° C. for 24 h. Put 0.45g of the powder obtained b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com