Liranaftate topical solution for treating fungal infection in pets and preparation method thereof
A technology for liranaftate and fungal infection, which is applied in the direction of medical preparations, antifungal agents, and pharmaceutical formulations of non-active ingredients, and can solve problems such as strong skin irritation, inconvenience to users, and obstacles, and achieve simple procedures, Ease of large-scale preparation, the effect of treating fungal infections in pets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 2
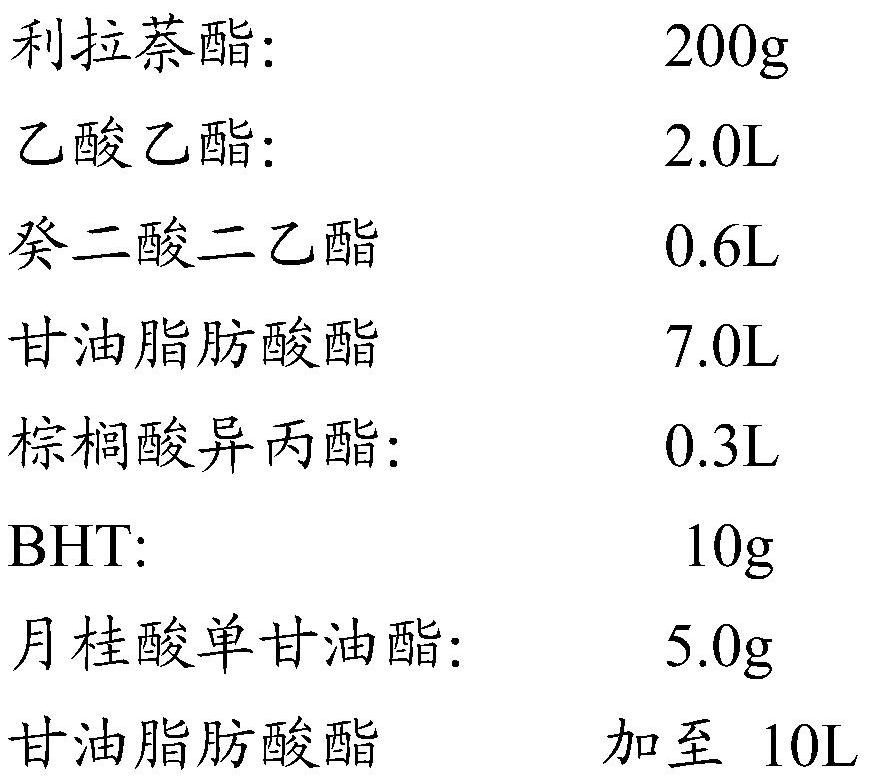
[0030] Preparation Example 2: Preparation of liranaphtate solution of the present invention
[0031] The components are as follows:
[0032]
[0033] Preparation method: In step A, at room temperature of 25°C, put the weighed liranaftyl ester, BHT, and BHA into the weighed ethyl acetate, diethyl sebacate, and appropriate amount of glycerin fatty acid ester, palm Stir in a stirring tank of isopropyl phosphate, the stirring speed range is 1000rpm / min, and the stirring time range is 30min, until it is completely dissolved, then add the remaining glycerin fatty acid ester to constant volume, and filter with a 0.45um nylon filter membrane to obtain a semi-finished product.
[0034] In step B, the above-mentioned semi-finished product is taken, filled and packaged.
Embodiment 3
[0035] The skin irritation research of embodiment 3 non-broken skin:
[0036] 3.1 Test items
[0037] Test drug:
[0038] Prepared by Luoyang Huizhong Veterinary Medicine Co., Ltd.; liranaphtate topical solution prepared in Example 1 of the present invention; liranaphtate topical solution prepared in Example 2 of the present invention; Japanese product ZEFNARTSOLUTION 2%.
[0039] Test animals:
[0040] 10 New Zealand white rabbits, weighing 2.5-3kg, half male and half male.
[0041] 3.2 Operation steps
[0042] Preparation of Rabbit Skin
[0043] Shave the hair on both sides of the rabbit’s back and spine 24 hours before administration. The shaved area on each side is about 3cm×3cm. Before administration, check whether the depilated skin is damaged due to depilation. Eight fur rabbits were used for the experiment.
[0044] Method of administration
[0045] Select 8 rabbits with intact skin after shaving, apply 0.5mL / only on the skin that has been shaved on the left sid...
Embodiment 4
[0067] Embodiment 4 damaged skin irritation research:
[0068] 4.1 Test items
[0069] Test drug:
[0070] Luoyang Huizhong Veterinary Medicine Co., Ltd.; liranaphtate topical solution prepared in Example 1 of the present invention; liranaphtate topical solution prepared in Example 2 of the present invention; Japanese product ZEFNARTSOLUTION 2%.
[0071] Test animals:
[0072] 10 New Zealand white rabbits, weighing 2.5-3kg, half male and half male.
[0073] 4.2 Test steps
[0074] Preparation of Rabbit Skin
[0075] 24 hours before the administration, the hair on both sides of the rabbit's back spine was shaved, and the shaved area on each side was about 3cm×3cm. Eight hairless rabbits were tested.
[0076] Method of administration
[0077] Select 8 rabbits with damaged skin after shaving, apply 0.5mL / only on the skin that has been shaved on the left side, and then cover with two layers of gauze (2.5cm×2.5cm) and a non-irritating plastic film , then fixed with non-irri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com