Application of Lignans Compounds in Preparation of Acaricide Drugs
A technology of lignans and compounds, applied in the field of pesticides, to achieve significant practical promotion value, significant social benefits, and good poisonous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
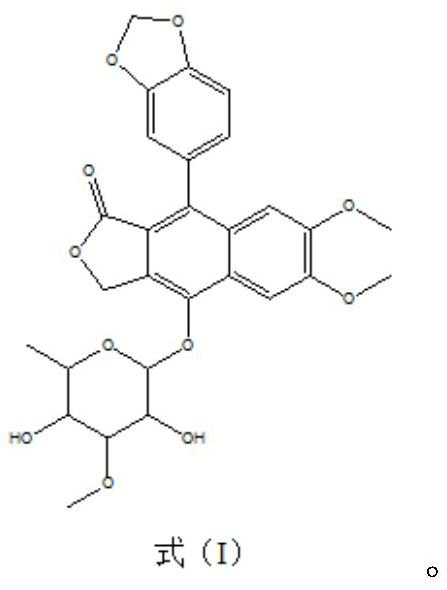
[0026] The acaricidal activity of embodiment 1 balusin to adult mite of Tetranychus urticae
[0027] The preparation of alopecia refers to the preparation method in the patent CN201811210133.6. The toxicity of alopecia to Tetranychus urticae was measured by spraying method. The specific method is as follows: acetone is first dissolved in acetone and prepared to a certain concentration mother liquor, and then diluted with 1% Tween aqueous solution to the required concentration; then inoculated the female adult mites of Tetranychus urticae on the kidney bean leaves in a petri dish covered with clean filter paper, sprayed with mites until the leaves are just covered with mist; after the mist evaporates and dries up, cover the dish and place it in the insect culture room for observation, regularly investigate the death situation, and calculate the corrected death rate according to the following formula. The blank control group (CK) was treated with the same solvent and emulsifier,...
Embodiment 2
[0033] Example 2 The acaricidal activity of balusin to nymphs of Tetranychus urticae
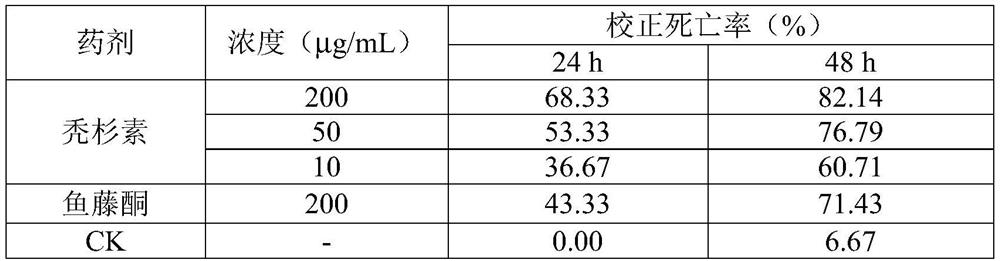
[0034] Determination of the biological activity of alopecia to nymphs of Tetranychus urticae, the test method refers to Example 1, and the measurement results are shown in Table 2.
[0035] Table 2 The activity of alopecia to nymphs of Tetranychus urticae
[0036]
[0037] Note: CK column is mortality rate.
[0038] It can be seen from Table 2 that after 10 μg / mL treatment, the 48-h corrected mortality rate of balusin on nymphs of Tetranychus urticae was above 70%, showing a significant acaricidal effect. There was a clear correlation between the mortality rate and the concentration, and the effect of 50 μg / mL baldoxine was greater than that of 200 μg / mL rotenone.
Embodiment 3
[0039] The acaricidal activity of embodiment 3 alopecia to Tetranychus cinnabarinus
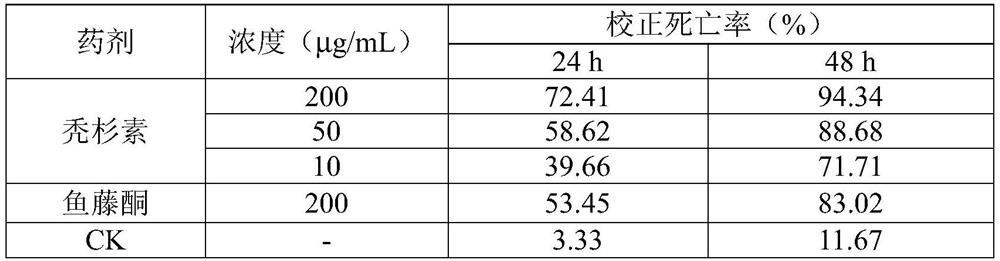
[0040] With reference to the method of Example 1, the acaricidal effect of balusin on Tetranychus cinnabarinus was measured, and the results are shown in Table 3.
[0041] Table 3 Toxicity of balusin to female adults of Tetranychus cinnabarinus
[0042]
[0043]
[0044] Note: CK column is mortality rate.
[0045] As can be seen from Table 3, baldrin also has acaricidal activity against Tetranychus cinnabarinus, and the difference in acaricidal activity between baldrin and rotenone at the same concentration (500 μg / mL) is obvious.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com