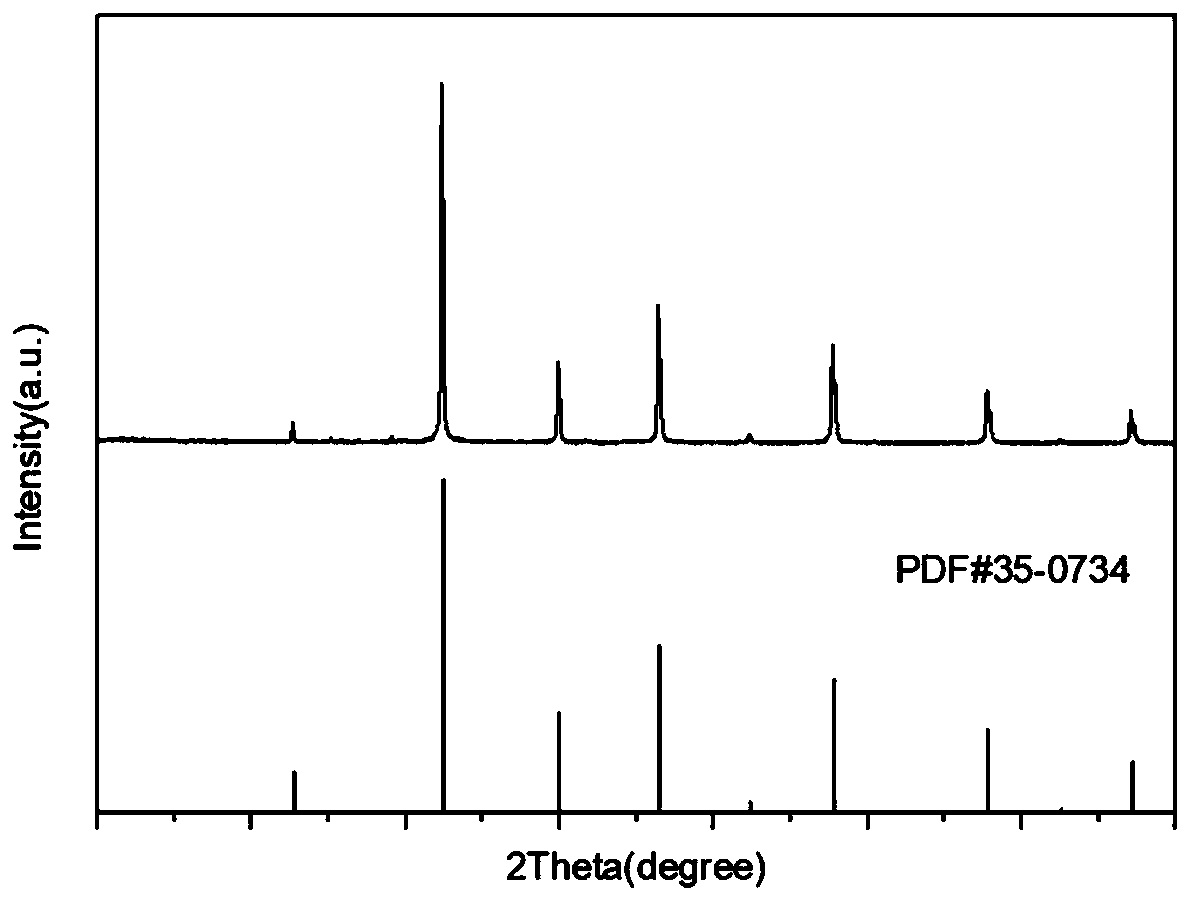
Preparation method of porous strontium titanate cubic particles doped with rare earth erbium ions
A technology of cubic particles and erbium ions, applied in the field of material science, can solve the problem of low utilization of sunlight, and achieve the effects of large specific surface area, adjustable band gap, and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Weigh a certain amount of titanium sulfate and dissolve it in deionized water, stir well to obtain a solution A with a concentration of 0.005mol / L; add an appropriate amount of potassium hydroxide solution dropwise to the above solution A, and stir to form a precipitate B; After the precipitate B was cleaned several times with ionized water, the dissolved strontium nitrate of 1mol / L, erbium nitrate with a doping content of 1mol%, and potassium hydroxide solution with a concentration of 6mol / L were added successively, and stirred until fully dissolved; The mixture was transferred to a reaction kettle, and hydrothermal reaction was carried out at 180° C. for 15 hours. Finally, the reaction product was taken out, filtered, washed with deionized water and ethanol, dried, and transferred to a blast drying oven at 80°C for 5 hours to obtain a porous strontium titanate cube doped with rare earth erbium ions. particles.
Embodiment 2
[0024] Weigh a certain amount of titanium sulfate and dissolve it in deionized water, stir well to obtain a solution A with a concentration of 0.005mol / L; add an appropriate amount of potassium hydroxide solution dropwise to the above solution A, and stir to form a precipitate B; After the precipitate B was cleaned several times with ionized water, the dissolved strontium nitrate of 1.2mol / L, erbium nitrate with a doping content of 10mol% and potassium hydroxide solution with a concentration of 10mol / L were added successively, and stirred until fully dissolved; The above mixture was transferred to a reaction kettle, and hydrothermal reaction was carried out at 200° C., and the reaction time was 4 hours. Finally, the reaction product was taken out, filtered, washed with deionized water and ethanol, dried, and transferred to a blast drying oven at 60°C for 10 hours to obtain a porous strontium titanate cube doped with rare earth erbium ions. particles.
Embodiment 3
[0026] Weigh a certain amount of titanium sulfate and dissolve it in deionized water, stir well to obtain a solution A with a concentration of 0.005mol / L; add an appropriate amount of potassium hydroxide solution dropwise to the above solution A, and stir to form a precipitate B; After the precipitate B was cleaned several times with ionized water, the dissolved strontium nitrate of 1.1mol / L, erbium nitrate with a doping content of 5mol%, and potassium hydroxide solution with a concentration of 8mol / L were added successively, and stirred until fully dissolved; The above mixture was transferred to a reaction kettle, and hydrothermal reaction was carried out at 190° C., and the reaction time was 10 h. Finally, the reaction product was taken out, filtered, washed with deionized water and ethanol, dried, and transferred to a blast drying oven at 70°C for 8 hours to obtain a porous strontium titanate cubic doped with rare earth erbium ions. particles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com