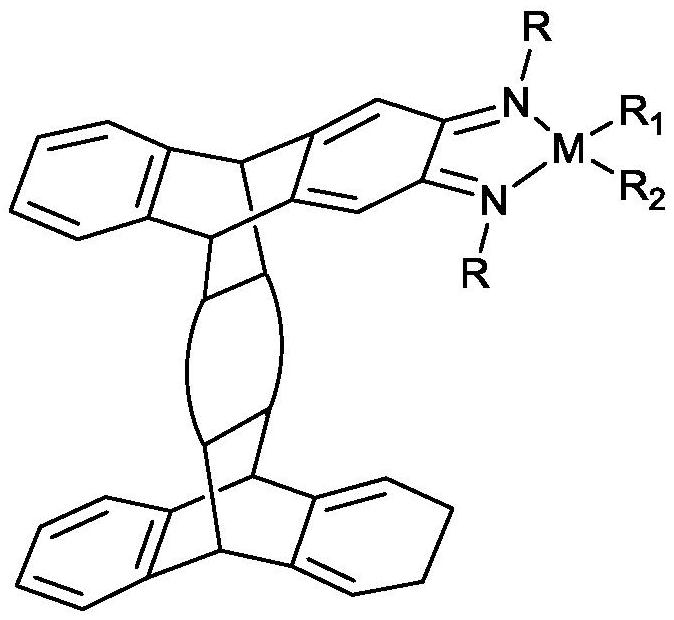
A kind of plate type α-diimine catalyst and its preparation method and application
A diimine and catalyst technology, which is applied in the field of plate-type α-diimine catalyst and its preparation, can solve the problems of high branching degree, poor catalytic performance, low molecular weight of polymer products, etc., and achieves low branching degree, The effect of low cost of raw materials and high molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Preparation of α-bis(2,6-diisopropyl)phenylimine ligand:
[0033] Add 100mL of toluene and 0.005mol of 5,7,12,14-tetrahydro-18λ with a plate structure into the reaction flask connected to the water separator 5 ,19λ 5 -5,14; 7,12-bis([1,2] endophenylene)pentacene-6,13,18,19-tetraketone and 0.020mol of 2,6-diisopropylaniline, heating Reaction at 120°C for 24h. After the reaction was completed, it was concentrated to 30 mL, placed in a refrigerator for crystallization, and the obtained crystal substance became a platy α-bis(2,6-diisopropyl)phenylimine ligand.
[0034] The obtained ligands were analyzed by NMR mass spectrometry, such as figure 1 shown.
[0035] It can be concluded from the figure that the structure of the ligand is as follows:
[0036] Same result as expected.
[0037] 2) Preparation of plate-type α-bis[(2,6-diisopropyl)phenylimine]palladium(II) catalyst
[0038] Add 30 mL of dichloromethane to the reaction flask, then add 0.005 mol of platy α-b...
Embodiment 2
[0042] 1) Preparation of platy α-bis(2,6-diisopropyl)phenylimine ligand:
[0043] Add 100mL of toluene and 0.005mol of 5,7,12,14-tetrahydro-18λ with a plate structure into the reaction flask connected to the water separator 5 ,19λ 5 -5,14; 7,12-bis([1,2] endophenylene)pentacene-6,13,18,19-tetraketone and 0.020mol of 2,6-diisopropylaniline, heating Reaction at 120°C for 72h. After the reaction was completed, it was concentrated to 30 mL, placed in a refrigerator for crystallization, and the obtained crystal substance became a platy α-bis(2,6-diisopropyl)phenylimine ligand.
[0044] 2) Preparation of plate-type α-bis[(2,6-diisopropyl)phenylimine]nickel(II) catalyst
[0045]Add 30 mL of dichloromethane to the reaction flask, followed by 0.005 mol of platy α-bis(2,6-diisopropyl)phenylimine ligand and 0.005 mol of (DME) 2 NiBr 2 , and reacted at 30°C for 1 hour. After the reaction, it was concentrated to 5 mL, and 20 mL of n-hexane was added for recrystallization and purificat...
Embodiment 3
[0049] 1) Preparation of α-diphenylimine ligand:
[0050] Add 100mL of toluene and 0.005mol of 5,7,12,14-tetrahydro-18λ with a plate structure into the reaction flask connected to the water separator 5 ,19λ 5 -5,14; 7,12-bis([1,2] endophenylene)pentacene-6,13,18,19-tetraketone and 0.020mol of aniline, heated to 120°C for 24h. After the reaction was completed, it was concentrated to 30 mL, placed in a refrigerator for crystallization, and the obtained crystalline substance became a platy α-diphenylimine ligand.
[0051] 2) Preparation of plate-type α-bis[(2,6-diisopropyl)phenylimine]palladium(II) catalyst
[0052] Add 30 mL of dichloromethane to the reaction flask, then add 0.005 mol of platy α-diphenylimine ligand and 0.005 mol of (COD)PdClMe, react at 30°C for 24 h, after the reaction, concentrate to 5 mL, 20 mL of n-hexane was added for recrystallization and purification, and the obtained powder solid was the plate-type α-diphenylimine palladium (II) catalyst.
[0053] 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com