Cobalt sulfide/layered double metal hydroxide composite electrocatalyst and preparation method thereof
A layered bimetallic, hydroxide technology, applied in catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc. Effect of electrocatalytic ability, increased specific surface area, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
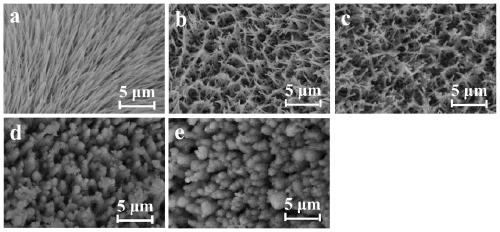
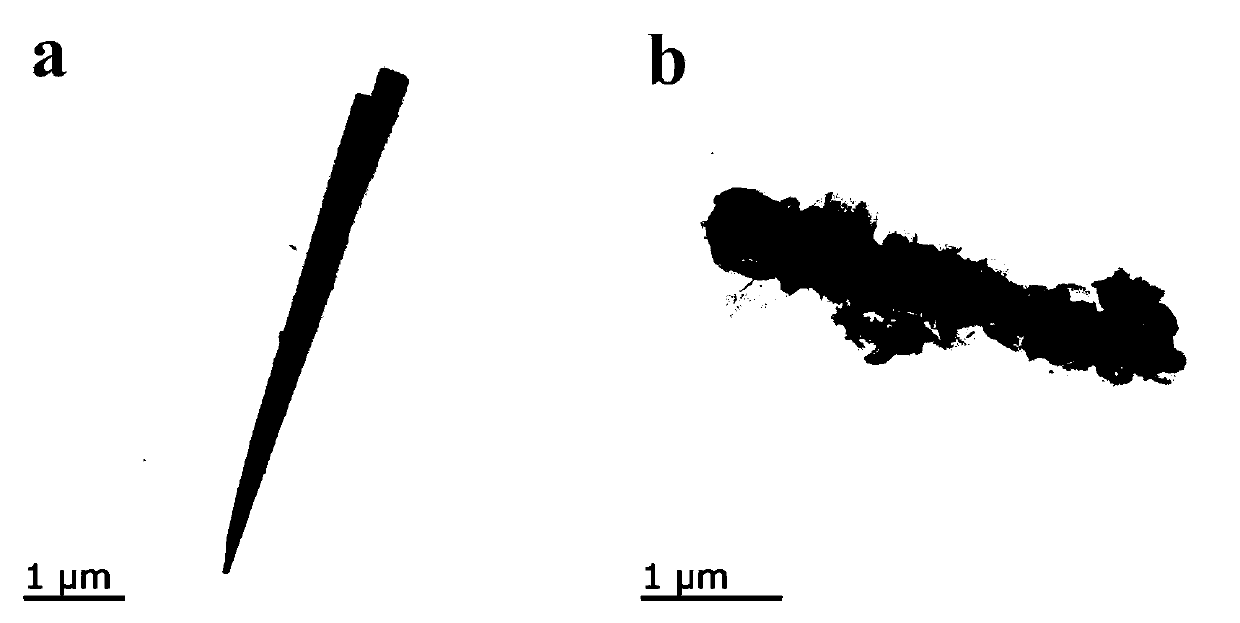
[0026] Example 1: Co 9 S 8 Preparation of / NF samples:
[0027] Weigh 1mmol of CoCl 2 ·6H 2 0 and 5 mmol of urea in a beaker, add deionized water to it, magnetically stir until completely dissolved, then add the resulting transparent pink solution to a polytetrafluoroethylene reactor, add pretreated foamed nickel to carry out hydrothermal reaction , 100°C, 6h; after naturally cooling to room temperature, the nickel foam was taken out, washed with water and alcohol several times, and dried to obtain Co(CO 3 ) 0.35 Cl 0.20 (OH) 1.10 Precursor samples. Weigh 0.25mmol Na 2 S, add deionized water to it, magnetically stir until completely dissolved, then add the obtained transparent solution into a polytetrafluoroethylene reaction kettle, add precursor samples for hydrothermal reaction, 100°C, 5h; after cooling to room temperature naturally , take out the nickel foam, wash and dry to get Co 9 S 8 / NF samples. Let cool and set aside.
Embodiment 2
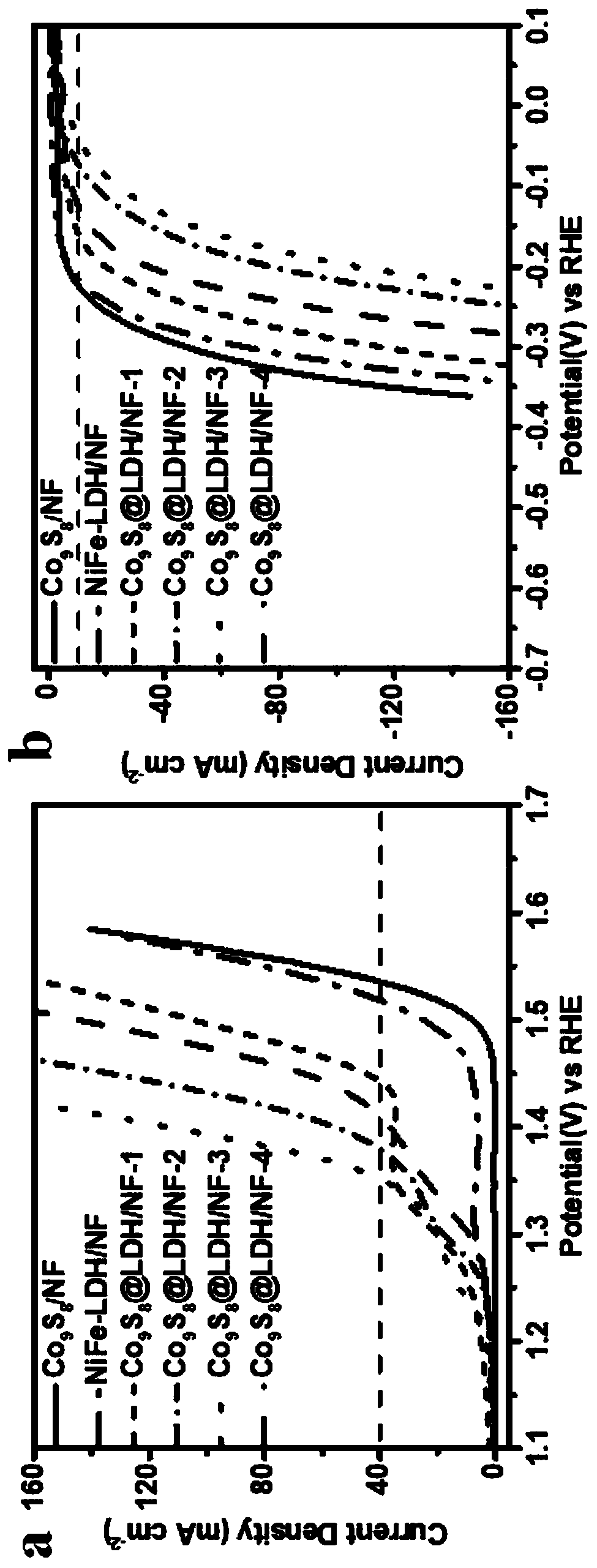
[0028] Example 2: Co 9 S 8 Preparation of @LDH / NF-1 sample (potential deposition 50s):
[0029] 0.15M Ni(NO 3 ) 2 ·6H 2 O and 0.15M FeSO 4 ·7H 2 O was placed in a beaker, and deionized water was added thereto, and magnetically stirred until it was completely dissolved to obtain a homogeneous solution A, which was set aside. The electrodeposition process is realized in the three-electrode system, first the Co obtained in Example 1 9 S 8 / NF as the working electrode, platinum wire and silver / silver chloride electrode as the counter electrode and reference electrode, respectively. At room temperature, the potential is -1.2 ~ -0.8V, and the A solution is used as the electrolyte, and the constant potential deposition is performed for 50s. After the deposition was completed, the nickel foam was taken out with tweezers, rinsed with deionized water and absolute ethanol, and dried to obtain Co 9 S 8 @LDH / NF Composite. The material is named Co 9 S 8 @LDH / NF-1.
Embodiment 3
[0030] Example 3: Co 9 S 8 Preparation of @LDH / NF-2 samples (potential deposition 100s):
[0031] 0.15M Ni(NO 3 ) 2 ·6H 2 O and 0.15M FeSO 4 ·7H 2 O was placed in a beaker, and deionized water was added thereto, and magnetically stirred until it was completely dissolved to obtain a homogeneous solution A, which was set aside. The electrodeposition process is realized in the three-electrode system, first the Co obtained in Example 1 9 S 8 / NF as the working electrode, platinum wire and silver / silver chloride electrode as the counter electrode and reference electrode, respectively. Under the condition of room temperature, the potential is -1.2~-0.8V, the A solution is used as the electrolyte, and the constant potential is deposited for 100s. After the deposition was completed, the nickel foam was taken out with tweezers, rinsed with deionized water and absolute ethanol, and dried to obtain Co 9 S 8 @LDH / NF Composite. The material is named Co 9 S 8 @LDH / NF-2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com