Staphylococcus lyase and preservation method and application thereof
A staphylococcus and preservation method technology, applied in the field of application, can solve the problems of drug resistance of phage lyase, difficult resistance of bacteria, activity reduction, etc., and achieve the effect of reducing dosage, improving bactericidal activity, and high expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
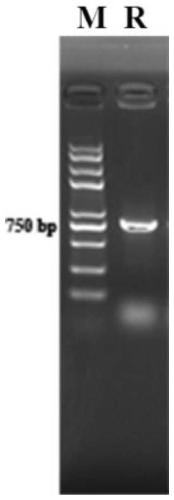
[0031] Embodiment 1: Construction of Staphylococcus lyase ClyC
[0032] Through literature review and experimental verification, we found a phage lyase binding domain protein with high affinity to Staphylococcus aureus peptidoglycan, and expressed it in fusion with another phage lyase catalytic domain protein with partial catalytic activity , to get a new staphylococcal phage lyase ClyC. The primers in this experiment were completed by Shanghai Sangong Company.
[0033] (1) The DNA sequence capable of expressing the cleavage enzyme ClyC was synthesized in Shanghai Sangon Bioengineering Co., Ltd. in its entirety. Using the ClyC gene as a template, NcoI and XhoI restriction sites were added to both ends of the target gene, and the primers were designed as follows:
[0034] SEQ.ID.NO.3 forward primer: TATACCATGGGCATGGCACTGCCTAAAACGG
[0035] SEQ.ID.NO.4 reverse primer: ATATCTCGAGCGCCCATTCGATGGTGCCCCAG
[0036]Using 2 μl of the gene as a template, add 1 μl of primers for PCR a...
Embodiment 2
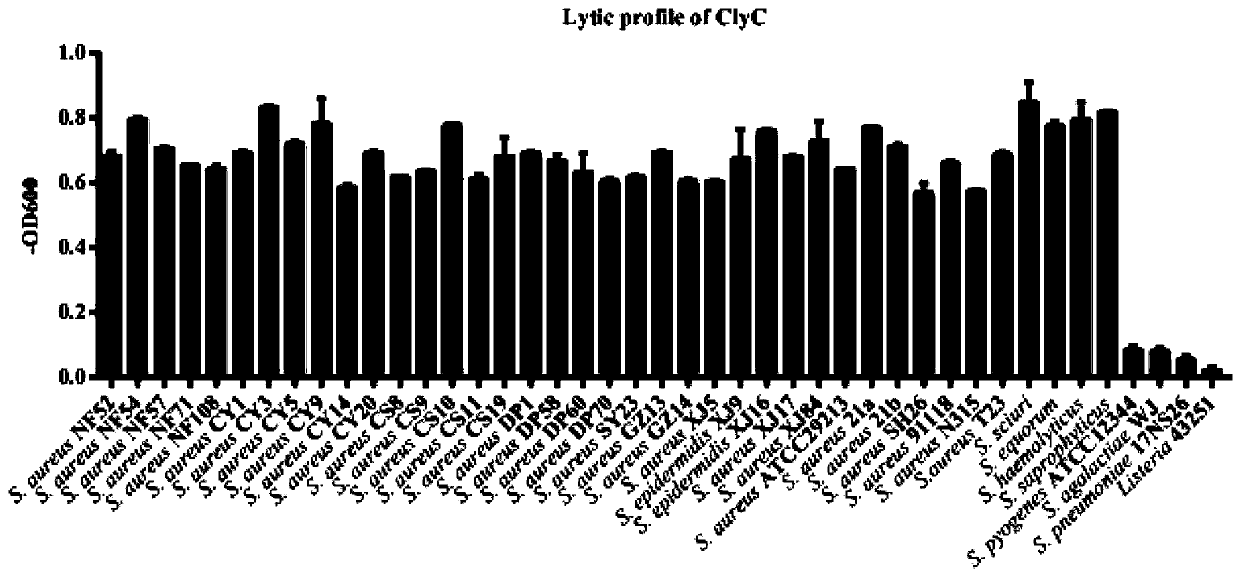
[0040] Example 2: ClyC kills a variety of staphylococci in vitro and specificity verification for different strains
[0041] In order to verify the bactericidal spectrum of ClyC, we selected several non-staphylococcus strains preserved in the inventor's laboratory and clinically isolated Staphylococcus aureus, Staphylococcus squirrel, Staphylococcus equus, Staphylococcus haemolyticus, Staphylococcus saprophyticus, Grape epidermidis Cocci and other strains were tested together. First, the strains to be tested were cultured overnight, collected by centrifugation, washed once with PBS, and then resuspended in PBS. Take a certain amount of ClyC and mix it with the above-mentioned bacterial solution, and at the same time use a microplate reader to detect the change in the absorption value of the mixture at 600 nm within 10 minutes. The lysis effect of different strains is indicated by the value of OD600 reduction. The killing effect obtained in the test is shown in figure 2 . ...
Embodiment 3
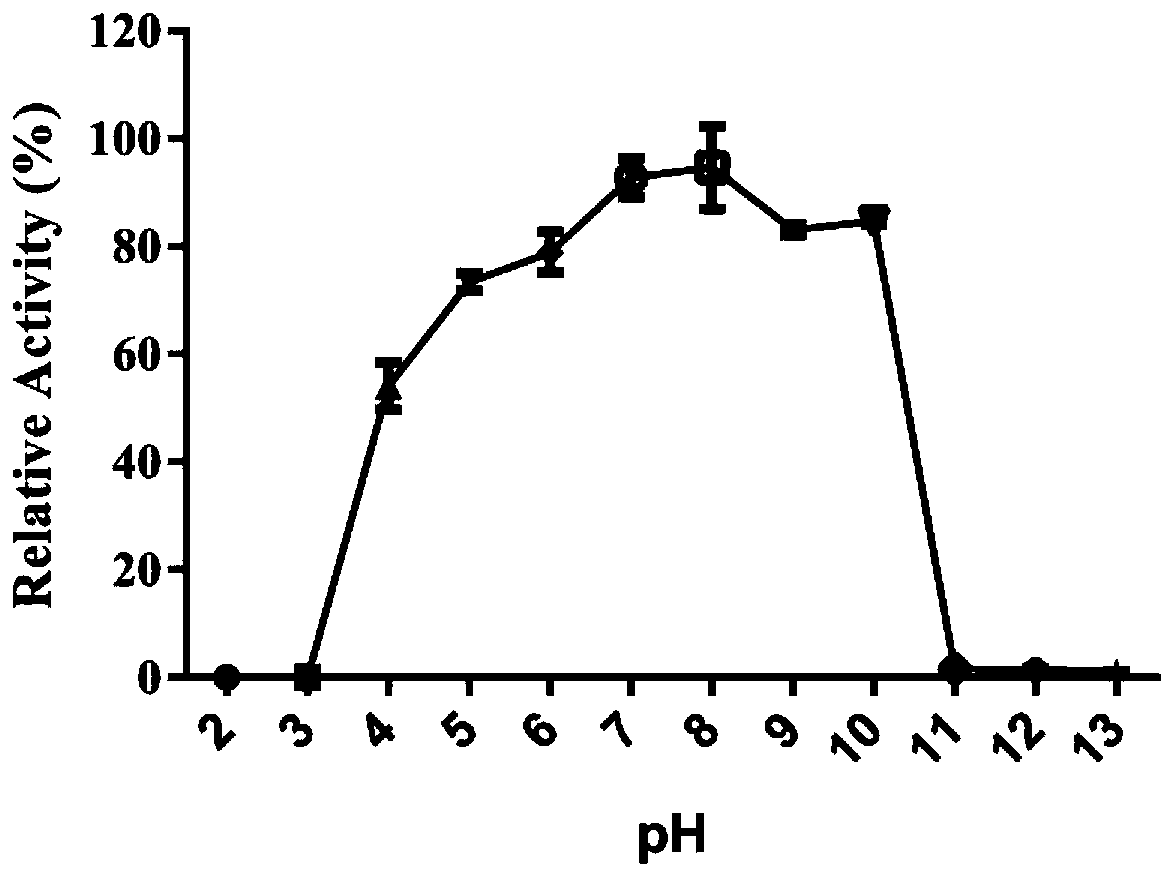
[0042] Example 3: Test of ClyC's ability to tolerate pH
[0043] The overnight culture of Staphylococcus aureus T23 was collected by centrifugation, washed once with PBS, and then dissolved in buffers with different pH values. Take a certain amount of ClyC and mix it with all the above bacteria solutions, and monitor the change of the absorption value of the mixed solution at 600nm with a microplate reader. After the detection, calculate the OD 600 reduction value of each group compared with the blank group, and define the value with the largest OD 600 reduction as 100%, and compare the OD 600 reduction values of other groups with it to obtain the relative enzyme activity value. For the relative activity of ClyC obtained at last, see image 3 . The results show that ClyC has good activity in the range of pH 4-10, and has high activity in pH 6-10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com