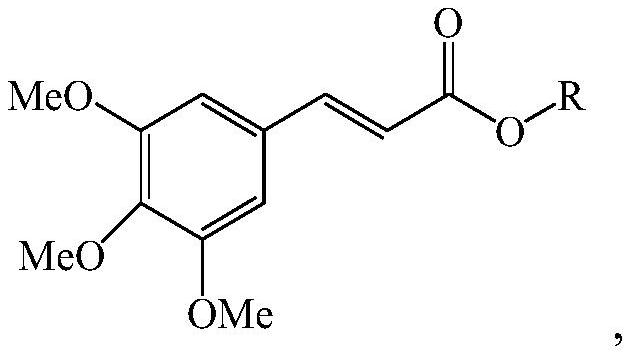
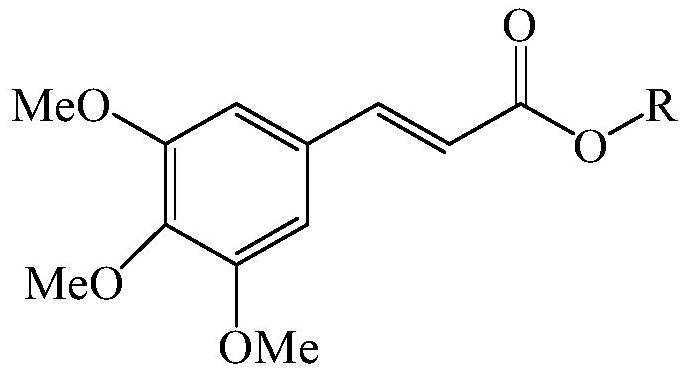
3,4,5-trimethoxycinnamate derivative, its preparation method and skin whitening composition containing the derivative
A technology of trimethoxycarnitine and trimethoxybenzenesulfonyl, applied in the field of new 3,4,5-trimethoxycinnamate derivatives, can solve the problem of lack of consumers, increase melanin, and increase the generation of skin free radicals Increase and other problems to achieve the effect of excellent skin whitening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: Preparation of (+)-3,4,5-trimethoxymenthyl cinnamate ((+)-menthyl 3,4,5-trimethoxycinnamate)
[0091] [chemical formula 2]
[0092]
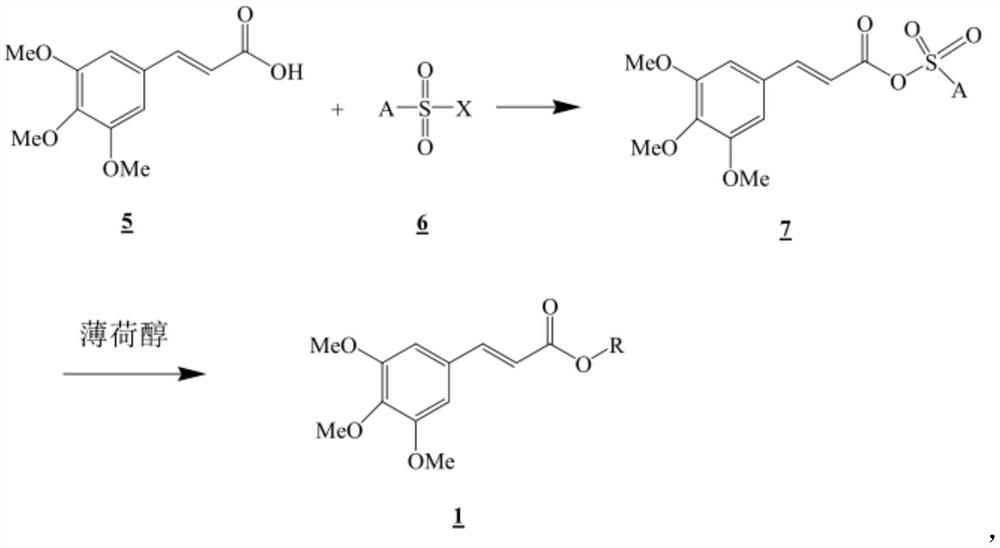
[0093] Dissolve 10 g of 3,4,5-trimethoxycinnamic acid (0.042 mol) in 100 mL of pyridine (pyridine), cool in an ice-water bath at 10°C, and then add 8.9 g (0.050 mol) of benzenesulfonate dropwise to it Acid chloride benzenesulfonyl chloride (benzenesulfonyl chloride) for 30 minutes. After slowly raising the reaction temperature to normal temperature and stirring for 2 hours, 6.5 g (0.042 mol) of 1R,2S,5R-(+)-menthol was dissolved in 30 mL of pyridine and added dropwise to the reaction solution for 3 minutes. After further stirring for 2 hours, the solvent was distilled off, and the residue was dissolved in 300 ml of ethyl acetate, after which the ethyl acetate solution was washed with 5% hydrochloric acid and distilled water, and magnesium sulfate and activated carbon were added thereto, followed by drying and decolorization...
Embodiment 2
[0096] Example 2: Preparation of (-)-3,4,5-trimethoxymenthyl cinnamate ((-)-menthyl 3,4,5-trimethoxycinnamate)
[0097][chemical formula 3]
[0098]
[0099] The target compound (11.5 g, 73%) was obtained as a white solid in the same manner as in Example 1 except that (-)-menthol was used instead of (+)-menthol.
[0100] LC (ethyl acetate:alkane=1:1) Rf=0.65
[0101] 1 H NMR (DMSO-d6, δ): 7.62 (d, 1H, J = 15.9Hz), 7.07 (s, 2H), 6.08 (d, 1H, J = 15.9Hz), 4.75 (m, 1H), 3.81 ( 3,6H),3.68(s,3H),1.88(m,2H),1.63(m,2H),1.43(m,2H),1.03(m,2H),0.91(m,6H),0.75(d ,3H,J=7.2Hz).
Embodiment 3
[0102] Example 3: Preparation of (+_)-3,4,5-trimethoxymenthyl cinnamate ((+_)-menthyl 3,4,5-trimethoxycinnamate)
[0103] [chemical formula 4]
[0104]
[0105] The target compound (11.0 g, 70%) was obtained as a white solid in the same manner as in Example 1 except that (±)-menthol was used instead of (+)-menthol.
[0106] TLC (ethyl acetate:alkane=1:1) Rf=0.65
[0107] 1 H NMR (DMSO-d6, δ): 7.62 (d, 1H, J = 15.9Hz), 7.07 (s, 2H), 6.08 (d, 1H, J = 15.9Hz), 4.75 (m, 1H), 3.81 ( 3,6H),3.68(s,3H),1.88(m,2H),1.63(m,2H),1.43(m,2H),1.03(m,2H),0.91(m,6H),0.75(d ,3H,J=7.2Hz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com