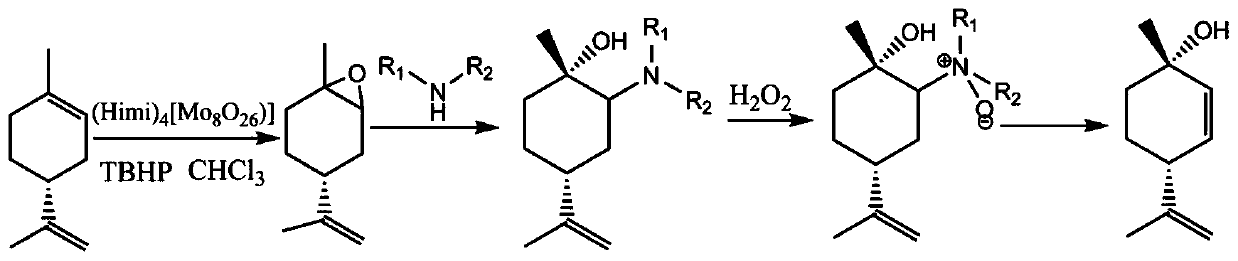
Preparation method of 1S,4R-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol
A technology of methyl vinyl and cyclohexene, applied in the field of medicine, can solve the problems of many processing steps, industrial production cost consumption, low yield and the like, and achieve the effects of high chiral purity, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: Add 136.23g limonene, 9.4g catalyst (Himi)4[(Mo8O26)], and 1.5L chloroform in sequence, and finally add 90g tert-butyl hydroperoxide as an oxidant, react at 45°C, and control the reaction , after the reaction was completed, filtered, and the filtrate quenched by sodium sulfite was extracted with chloroform, dried, and the solvent was distilled off under reduced pressure to obtain 144 g of intermediate 1 with a yield of 94%.
[0030] Step 2: Add 140g of intermediate 1 to the reaction flask, then add 517g of dimethylamine aqueous solution (40%), reflux for 18 hours, extract with methyl tert-butyl ether, dry, and concentrate to obtain 80g of intermediate 2, yield 88%.
[0031] Step 3: Add 80g of intermediate 2 and 1.6L of water in sequence in the reaction bottle, add 406ml of hydrogen peroxide dropwise under ice bath, remove the ice bath after dropping, and control the reaction in the middle. After the reaction is over, quench the reaction with sodium bisulfite . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com