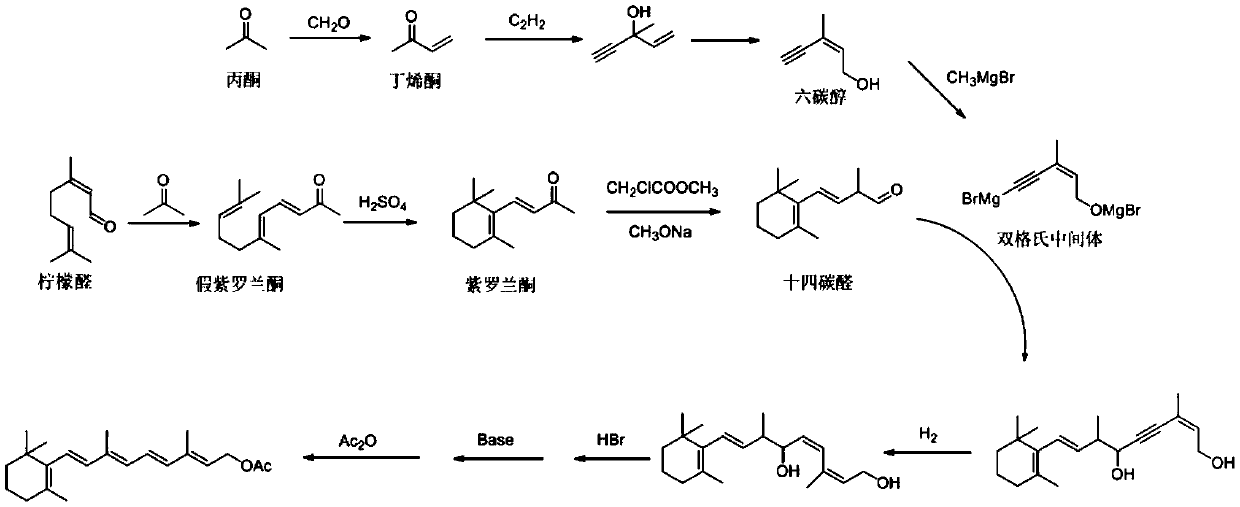
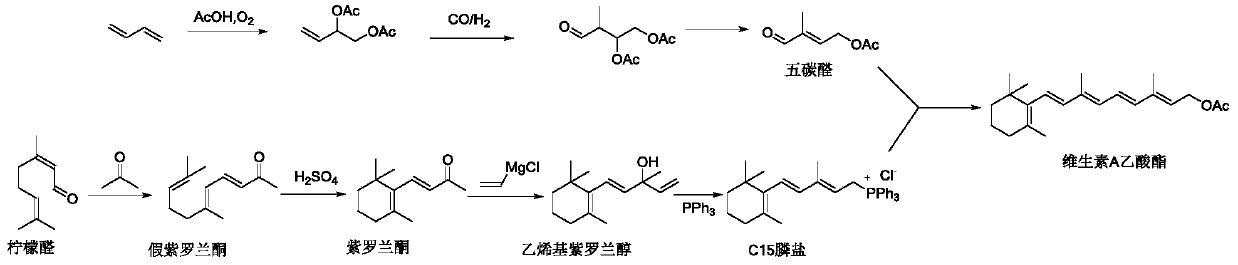
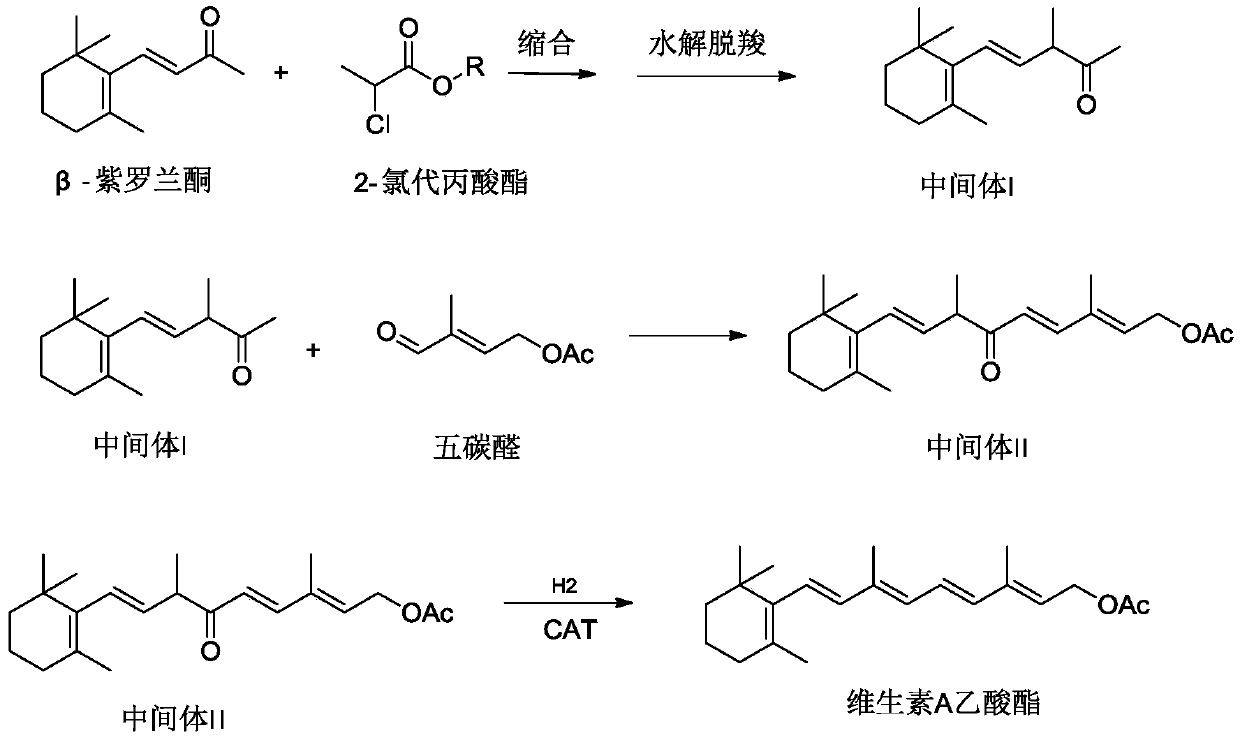
Preparation method of retinyl acetate
A technology of acetate and vitamin, applied in the field of preparation of vitamin A acetate, can solve the problems of long reaction steps, difficult handling, safety problems in the production of double Grignard reagents, etc., and achieves the effects of mild reaction conditions and avoiding three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In a 2L three-necked flask, 200g (1.0mol) of β-ionone and 150g (1.2mol) of methyl 2-chloropropionate were mixed, cooled to -40°C, and 280ml of 30%wt sodium methoxide methanol solution was added dropwise ( 1.4mol), the temperature should not exceed -35°C. After the dropwise addition, react at -40°C to -35°C for 4 hours. The cooling bath was removed, and the temperature in the system was naturally raised to 10°C. Add 500g of water and hydrolyze at 40-45°C for 30 minutes. After standing still, the oil layer was separated; the water layer was extracted with petroleum ether 300mL×3. The extract was combined with the oil layer and washed with 1% aqueous acetic acid until the pH reached 7. After recovering petroleum ether, distill under reduced pressure, collect 110 ℃ ~ 130 ℃ (absolute pressure 100Pa) fraction, which is intermediate I197g (0.85mol), its content is 95% according to gas chromatography, it is a yellow oil, and the yield is 85% . Intermediate I is qualitative...
Embodiment 2
[0055] In a 3L three-necked flask, 200g (1.0mol) of β-ionone and 209g (1.5mol) of ethyl 2-chloropropionate were mixed, cooled to -25°C, and 650ml of 18%wt sodium ethylate ethanol solution was added dropwise ( 1.5mol), the temperature should not exceed -20°C. After the dropwise addition, react at -25°C to -20°C for 3 hours. The cooling bath was removed, and the temperature in the system was naturally raised to 10°C. Add 800g of water and hydrolyze at 40-45°C for 30 minutes. After standing, the oil layer was separated; the water layer was extracted with petroleum ether 400mL×3. The extract was combined with the oil layer and washed with 1% aqueous acetic acid until the pH reached 7. After recovering petroleum ether, distill under reduced pressure, collect 110 ℃ ~ 130 ℃ (absolute pressure 100Pa) fraction, which is intermediate I194g (0.84mol), its content is 95% according to gas chromatography, it is a yellow oil, and the yield is 84% .
[0056] Add 600g of methylene chlorid...
Embodiment 3
[0059] In a 3L three-necked flask, mix 200g (1.0mol) of β-ionone and 277g (1.8mol) of isopropyl 2-chloropropionate, cool to 0°C, and add dropwise 1000ml of 2mol / L potassium tert-butoxide solution (2.0mol), the temperature is controlled not to exceed 5°C. After the dropwise addition, react at 0°C to 5°C for 2 hours. The cooling bath was removed, and the temperature in the system was naturally raised to 10°C. Add 800g of water and hydrolyze at 40-45°C for 30 minutes. After standing, the oil layer was separated; the water layer was extracted with petroleum ether 400mL×3. The extract was combined with the oil layer and washed with 1% aqueous acetic acid until the pH reached 7. After recovering petroleum ether, distill under reduced pressure, collect 110°C-130°C (absolute pressure 100Pa) fraction, which is intermediate I206g (0.89mol), its content is 95% by gas chromatography, it is a yellow oil, and the yield is 89% .
[0060] Add 600g of isopropanol, 154g of five-carbon alde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com