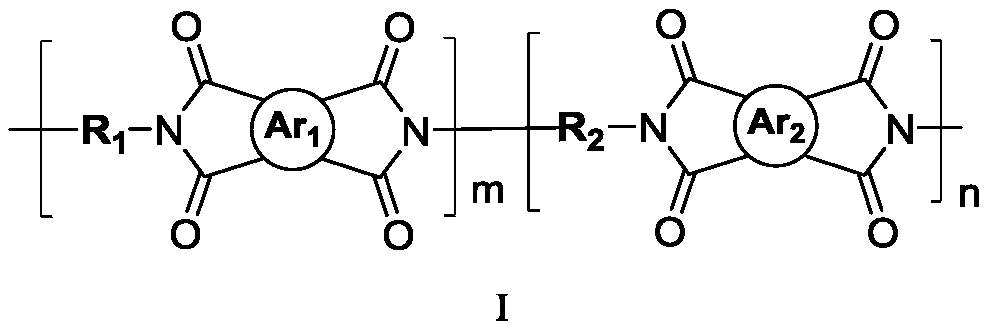
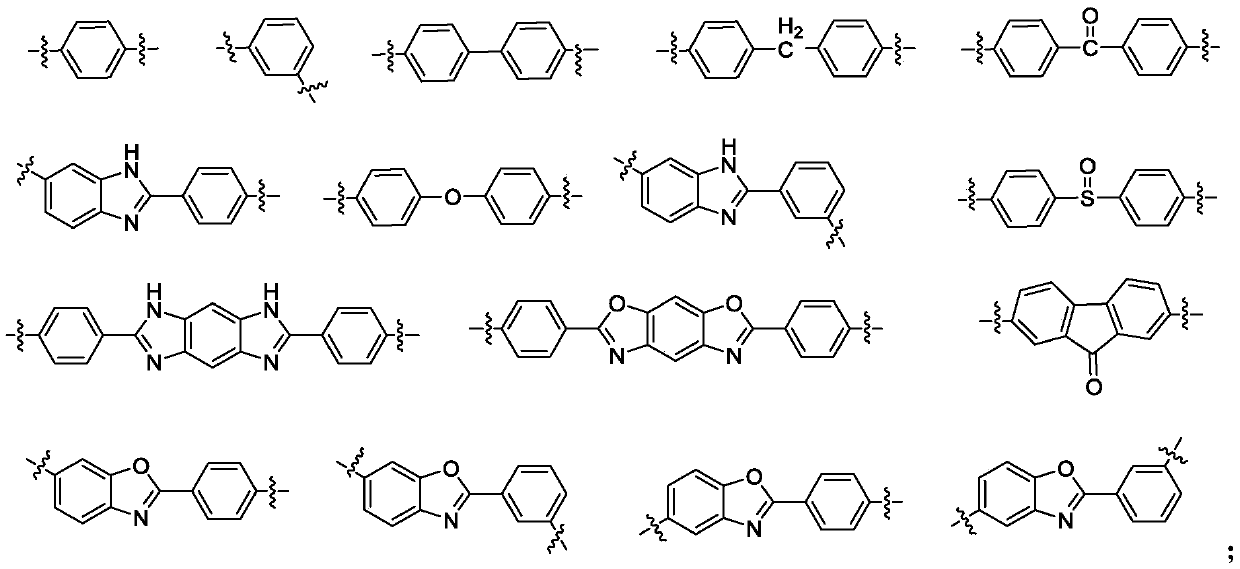
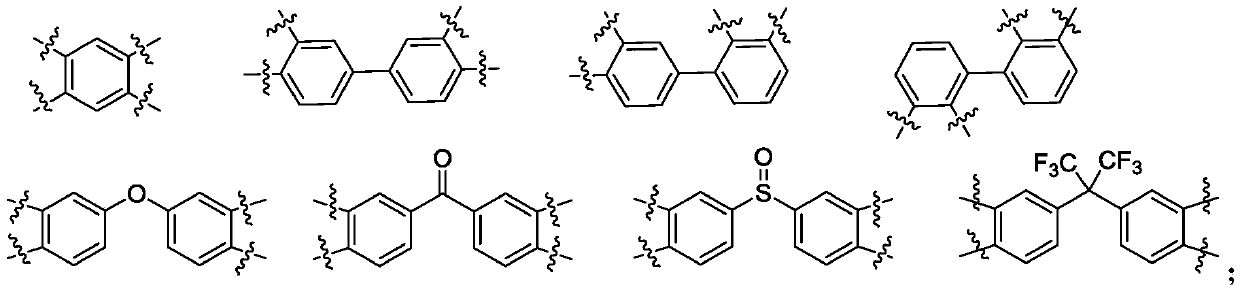
Polyimide with high thermal dimensional stability, preparation method therefor and application of polyimide
A technology of polyimide and polyamic acid, which is applied in the field of high molecular polymers, can solve the problems that cannot satisfy the processing and application of flexible display substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1. N, N'-bis(5-nitropyridin-2-yl) p-benzamide
[0101]
[0102] Add 30.0g of 5-nitro-2-aminopyridine and 150mL of pyridine into a 500mL two-necked flask, and stir at room temperature for 5min until the solid dissolves. Dissolve 21.89g of terephthaloyl chloride in 100mL of tetrahydrofuran, and slowly drop it into the two-necked flask within 2 hours. After stirring and reacting at room temperature for 36 h, the precipitated solid was filtered, washed with water, and refluxed in 300 mL of methanol for 1 h. Then the solid was filtered and dried to obtain 34.5 g of white solid with a yield of 78%. 1 H NMR (400MHz, CDCl 3 , ppm): δ11.73(s,2H), 9.22(d,2H), 8.66(dd,2H), 8.41(dd,2H), 8.13(d,4H). 13 C NMR (151MHz, CDCl 3 , ppm): δ 163.95, 152.05, 141.33, 139.62, 133.72, 129.47, 124.89, 114.79.
Embodiment 2
[0103] Example 2. N, N'-bis(5-aminopyridin-2-yl) p-benzamide
[0104]
[0105] Add 25.0g of N,N'-bis(5-nitropyridin-2-yl)p-benzamide and 100mL of N,N'-dimethylacetamide into a 500mL two-neck flask, stir at 80°C for 30min until the solid dissolves . Add 3g of 10wt% Pd / C, 50mL of methanol to the bottle, slowly add 40mL of 80wt% hydrazine hydrate dropwise, and release gas. After no more gas is released, stir at 80°C for 6h. Pd / C was filtered off with celite to obtain a clear yellow filtrate, which was concentrated and poured into acetone. The precipitated solid was filtered, refluxed repeatedly in acetone, and dried to obtain 18 g of a yellow solid with a yield of 85%. 1 H NMR (400MHz, CDCl 3, ppm): δ10.47(s,2H),8.02(s,4H),7.79(d,2H),7.72(d,2H),6.99(dd,2H),5.18(s,4H). 13 C NMR (101 MHz, DMSO-d 6 , ppm) 164.60, 142.53, 142.07, 137.37, 133.75, 128.05, 122.65, 116.42.
Embodiment 3
[0106] Example 3.4-nitro-N-(5-nitropyridin-2-yl)benzamide
[0107]
[0108] Add 20.0g of 5-nitro-2-aminopyridine to a 500mL two-necked flask, 150mL of pyridine, and stir at room temperature for 5min until the solid dissolves. 26.68g of 4-nitrobenzoyl chloride was dissolved in 100mL of tetrahydrofuran, and slowly added dropwise into the two-necked flask within 2h. After stirring and reacting at room temperature for 36 h, the precipitated solid was filtered, washed with water, and refluxed in 300 mL of methanol for 1 h. Then the solid was filtered and dried to obtain 31.5 g of a white solid with a yield of 76%. 1 H NMR (400MHz, CDCl 3 ,ppm): δ11.89(s,1H),9.22(d,1H),8.66(dd,1H),8.41(d,1H),8.31(d,2H),8.20(d,2H). 13 C NMR (101MHz, CDCl 3 , ppm): δ152.76, 145.33, 140.08, 136.13, 132.72, 131.72, 127.90, 124.38, 119.20, 111.89.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com