Pharmaceutical composition containing fesoterodine and preparation method of pharmaceutical composition
A technology of fesoterodine and its composition, which is applied in the field of medicinal chemistry, can solve problems such as gastrointestinal irritation, shortened shelf life, osmotic diarrhea, etc., and achieve the effects of prolonging storage time, enhancing stability, and slowing down oxidative degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
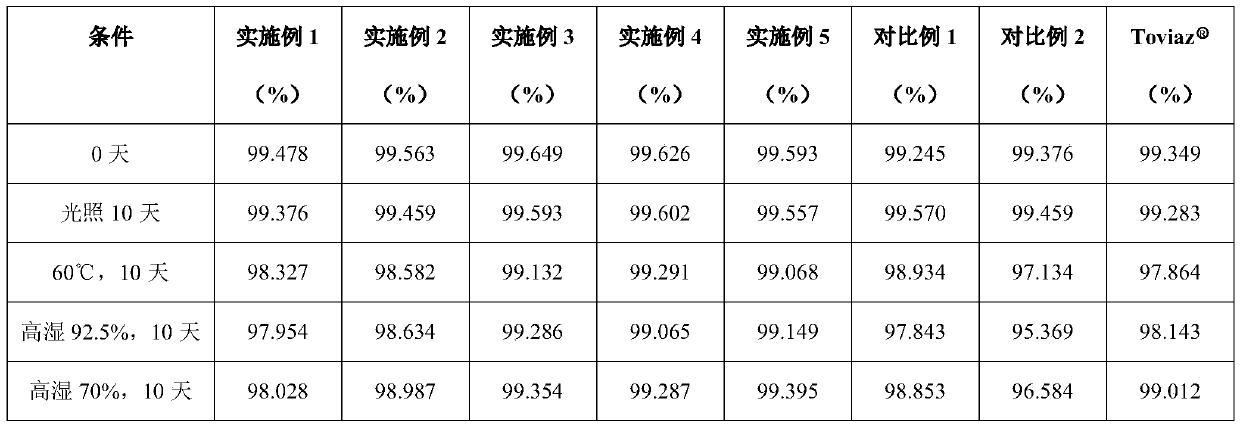
Examples
Embodiment 1
[0071] (1) Preparation of Fesoterodine oily suspension product
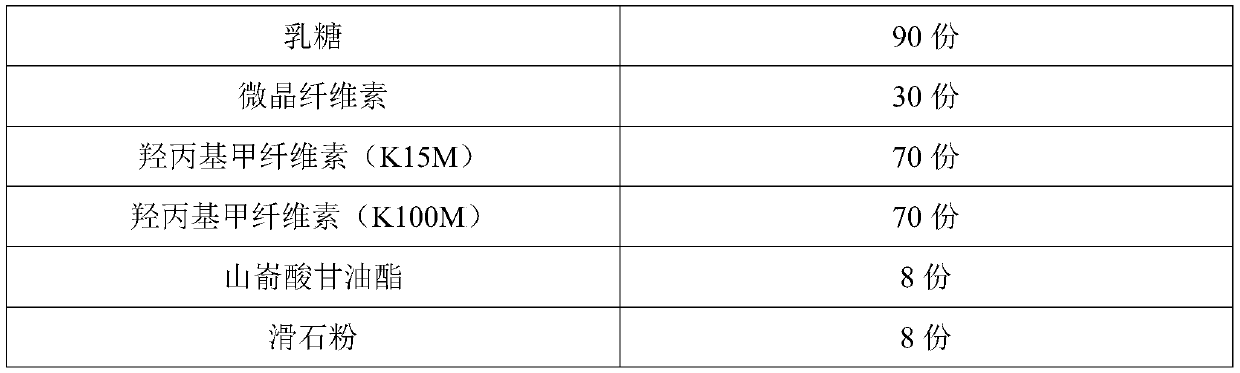
[0072] Put 300mL soybean oil into a reaction container, put it in a vacuum drying oven for 5 minutes, take it out, seal it, and ultrasonicate it at room temperature for 5 minutes for pre-dispersion. Ultrasonic pre-dispersion of soybean oil can make the coated oily film more uniform and fine , can improve the stability of the follow-up fesoterodine fumarate oily suspension product and can also reduce the amount of surfactant used. Keep filling with nitrogen in the reaction vessel that soybean oil is housed, cut off air and enter, add 10g fesoterodine fumarate and 1g monoglyceride diacetyl tartrate in reaction vessel, make fesoterodine fumarate and diacetyl tartrate The weight ratio of acetyl tartrate monoglyceride is 10:1, and then stir and disperse at 500 rpm for 10 hours to obtain the semi-finished product of fesoterodine fumarate oily suspension, and then mix the obtained fesoterodine fumarate oily suspension ...
Embodiment 2
[0079] (1) prepare fesoterodine oily suspension product
[0080] Put 300mL of olive oil into a reaction vessel, put it in a vacuum drying oven for 30 minutes, take it out, seal it and ultrasonicate it at room temperature for 10 minutes for pre-dispersion, and keep filling nitrogen in the reaction vessel with olive oil. Add 5g of fesoterodine fumarate and 0.1g of propylene glycol fatty acid ester to the container, so that the weight ratio of fesoterodine fumarate and propylene glycol fatty acid ester is 5:0.1, then stir and disperse at a speed of 3000rpm for 3h, Obtain the semi-finished product of fesoterodine fumarate oily suspension, then centrifuge the semi-finished product of fesoterodine fumarate oily suspension obtained at 5000rpm, remove the clarified oil layer after centrifugation, then wash 2 times with ethanol, wash with Afterwards, the semi-finished product of fesoterodine oily suspension was obtained and vacuum-dried at 25°C to finally obtain the fesoterodine oily s...
Embodiment 3
[0086] (1) prepare fesoterodine oily suspension product
[0087] Put 300mL of a mixture of linseed oil and soybean oil into a reaction vessel, wherein the weight ratio of linseed oil and soybean oil is 2:0.8, then add 0.6g of soybean lecithin, put it in a vacuum drying oven for 30 minutes, and take it out Seal and ultrasonicate at room temperature for 5min to carry out pre-dispersion, keep filling with nitrogen in the reaction vessel equipped with linseed oil and soybean oil, add 10g fesoterodine fumarate and 0.3g citrate monoglycerol in the reaction vessel ester, so that the weight ratio of fesoterodine fumarate and monoglyceride citrate is 10:0.3, then stir and disperse for 5h at a rotating speed of 3000rpm to obtain the semi-finished product of fesoterodine fumarate oily suspension, and then Obtain the fesoterodine fumarate oily suspension semi-finished product and carry out centrifugation under 5000rpm, remove the clarified oil layer after centrifugation, then wash 2 times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com