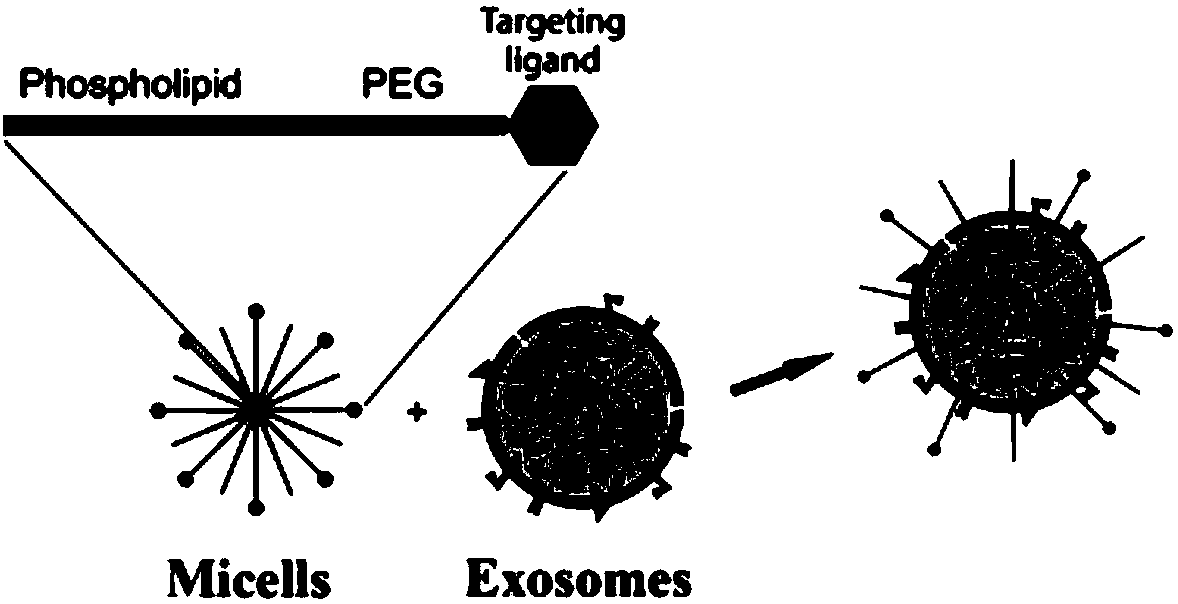
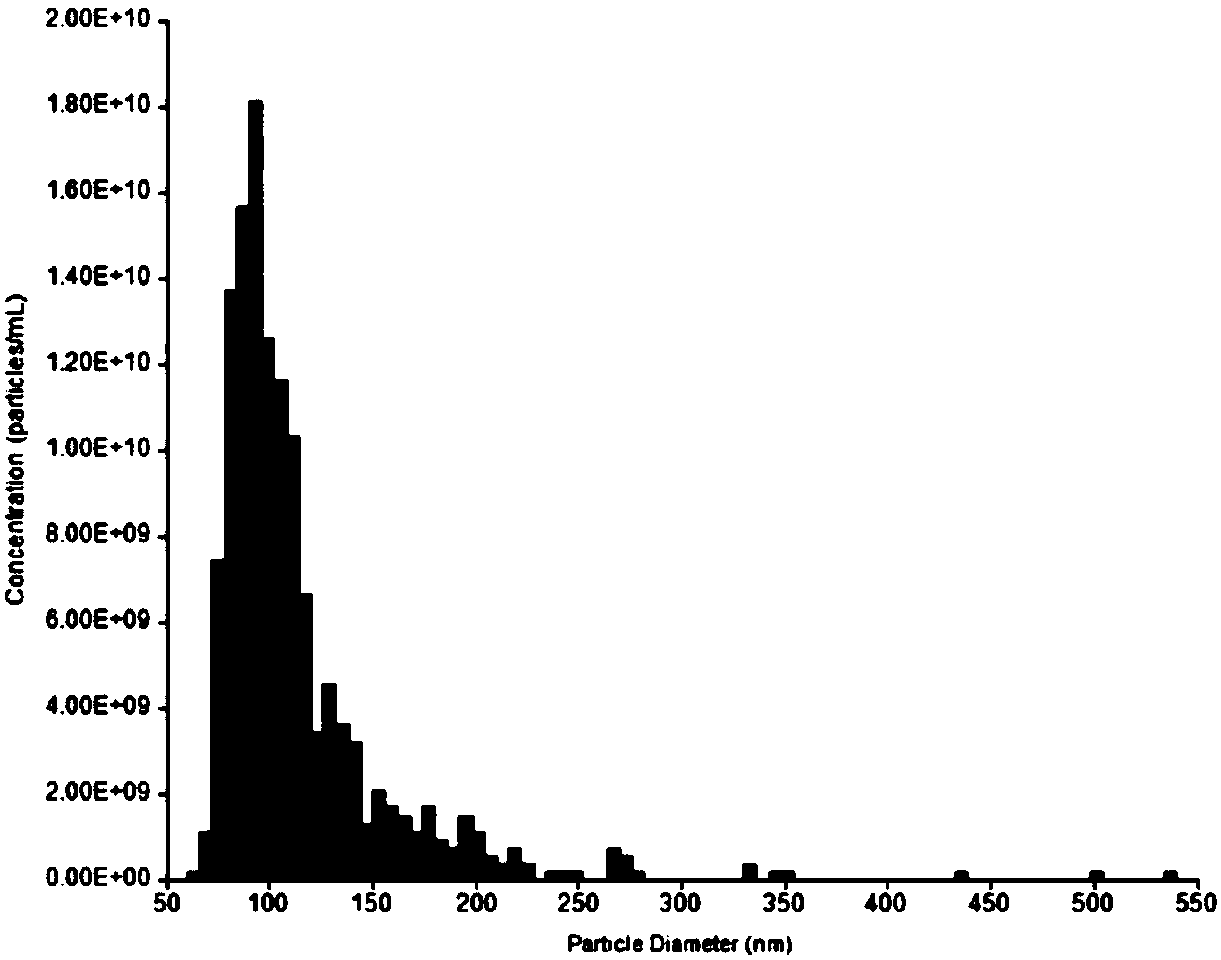
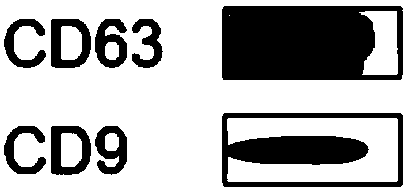
Human pluripotent stem cell exosomes loaded with antitumor drugs and preparation method and use of human pluripotent stem cell exosomes
A technology of human pluripotent stem cells and anti-tumor drugs, applied in non-embryonic pluripotent stem cells, artificially induced pluripotent cells, anti-tumor drugs, etc., can solve the problems that the treatment potential of diseases and injuries has not been fully utilized and needs further confirmation , to achieve the effect of reducing dosage and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Culture of human embryonic stem cells (ESCs) and extraction and identification of exosomes
[0122] A layer of embryonic stem cell Matrigel (ESC-Qualified BD Matrigel, BD Sparks, MD, USA), ESCs were moved into the dish, and mTeSR1 serum-free medium (StemCell Vancouver, BC, Canada), cultivated in an incubator (37° C., 5% CO2, saturated humidity), and collected the culture medium changed every day. Filter the medium through a 0.22 micron pore size filter membrane and centrifuge at 10,000g at 4°C for 30 minutes to remove cell debris; use a 100KD molecular weight ultrafiltration tube and centrifuge (3500g, 15min) to intercept exosomes in the concentrated supernatant to obtain exosomes Concentrate; transfer the concentrate to 30% sucrose / heavy water density pad (1.210g / cm3), centrifuge at 100000g at 4°C for 210 minutes, collect the bottom 5ml sucrose / heavy water density pad, add PBS to dilute, and transfer to an ultra-thin filter with a molecular weight cut-off of 100KD ...
Embodiment 2
[0127] Culture of human induced pluripotent stem cells (iPSCs) and extraction and identification of exosomes
[0128] A layer of embryonic stem cell Matrigel (ESC-Qualified BD Matrigel, BD Sparks, MD, USA), iPSCs were moved into the dish, and mTeSR1 serum-free medium (StemCell Vancouver, BC, Canada), cultivated in an incubator (37° C., 5% CO2, saturated humidity), and collected the culture medium changed every day. Filter the medium through a 0.22 micron pore size filter membrane and centrifuge at 10,000g at 4°C for 30 minutes to remove cell debris; use a 100KD molecular weight ultrafiltration tube and centrifuge (3500g, 15min) to intercept exosomes in the concentrated supernatant to obtain exosomes concentrate; transfer the concentrate to a 30% sucrose / heavy water density pad (1.210 g / cm 3 ), centrifuge at 100,000g at 4°C for 210 minutes, collect the 5ml sucrose / heavy water density pad at the bottom, add PBS to dilute, transfer to an ultrafiltration centrifuge tube with a...
Embodiment 3
[0133] Human ESC-derived exosomes (ESC-Exos) encapsulated paclitaxel (PTX) by co-incubation method
[0134] ESC-Exos solution is from Example 1. Quantitative standard curve determination of PTX: Accurately weigh 1mg of PTX powder and dissolve it in 1mL of methanol, then dilute the solution with methanol to configure methanol standard solutions of 50μg / mL, 20μg / mL, and 10μg / mL of PTX, HPLC injection. The test conditions are as follows: flow rate 1mL / min; injection volume 10μL; detection wavelength 227nm; column temperature 30°C, separation column: Zorbax Extend-C18Analytical, 4.6×150mm 5-micron.
[0135] After obtaining the corresponding experimental results, the peak area (PA) of the chromatographic peak was plotted as a function of the PTX concentration (C, μg / mL) (see attached Figure 6 shown), obtain the quantitative standard curve of Res under the following chromatographic separation conditions: PA=26.7388C+2.6119
[0136] Encapsulation of PTX: prepare 1 mg / mL PTX in ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com