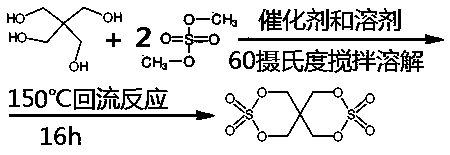
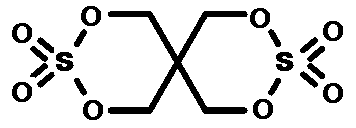Preparation method for synthesizing pentaerythritol bicyclic sulfate
A technology of pentaerythritol and synthesis method, which is applied in the direction of organic chemistry, etc., can solve the problems such as the influence of battery electrical performance, great harm, and poor safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 252g of dimethyl sulfate, 0.1g of catalyst titanate, and 500g of benzene solvent into a 2L four-necked bottle at room temperature, pass through nitrogen protection, stir and heat up to 60 degrees to completely dissolve, and continue to heat up to 120 degrees after the dissolution is completed. Add pentaerythritol in batches within 10 hours. Since pentaerythritol is an insoluble white powder, pentaerythritol will disappear only through reaction. Observe the disappearance of pentaerythritol every time to judge the reaction termination point. Methanol will be produced during the reaction, and it will be fractionated by circulating nitrogen and condensation The methanol gas is taken out of the reactor for recovery. When 136~200g of pentaerythritol is added, no powder disappears, indicating that the reaction has reached the end point, and the excess pentaerythritol is cooled and filtered, and then rectified to obtain 3,3,9,9-tetraoxo-2,4,8,10-tetraoxo- The crude product ...
Embodiment 2
[0022] Add 504g of dimethyl sulfate, 0.1g of catalyst p-toluenesulfonic acid, and 800g of benzene solvent into a 3L four-necked bottle at normal temperature, and pass through nitrogen protection, stir and heat up to 80 degrees to completely dissolve, and continue to heat up to 150 degrees after the dissolution is completed. Add pentaerythritol in batches within 10 hours. Since pentaerythritol is an insoluble white powder, pentaerythritol will only disappear through reaction. Observe the disappearance of pentaerythritol every time to judge the end point of the reaction. Methanol will be produced during the reaction. Through circulating nitrogen and condensation Fractional distillation recovers methanol. When 272~400g of pentaerythritol is added, no powder disappears, indicating that the reaction has reached the end point, and the excess pentaerythritol is cooled and filtered, and then rectified to obtain 3,3,9,9-tetraoxo-2,4,8,10-tetraoxo- The crude product of 3,9-dithiaspiro[5...
Embodiment 3
[0024] Add 252g of dimethyl sulfate, 0.1g of catalyst titanium sulfate, and 500g of benzene solvent into a 2L four-necked bottle at normal temperature, pass through nitrogen protection, stir and heat up to 80 degrees to completely dissolve, and continue to heat up to 150 degrees after the dissolution is completed. Add pentaerythritol in batches within an hour. Since pentaerythritol is an insoluble white powder, pentaerythritol will only disappear through reaction. Observe the disappearance of pentaerythritol every time to judge the reaction termination point. Methanol will be produced during the reaction, and it will be recovered by circulating nitrogen and condensation fractionation Methanol. When 130~200g of pentaerythritol is added, no powder disappears, indicating that the reaction has reached the end point, and the excess pentaerythritol is cooled and filtered, and then rectified to obtain 3,3,9,9-tetraoxo-2,4,8,10-tetraoxo- The crude product of 3,9-dithiaspiro[5.5]undeca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com