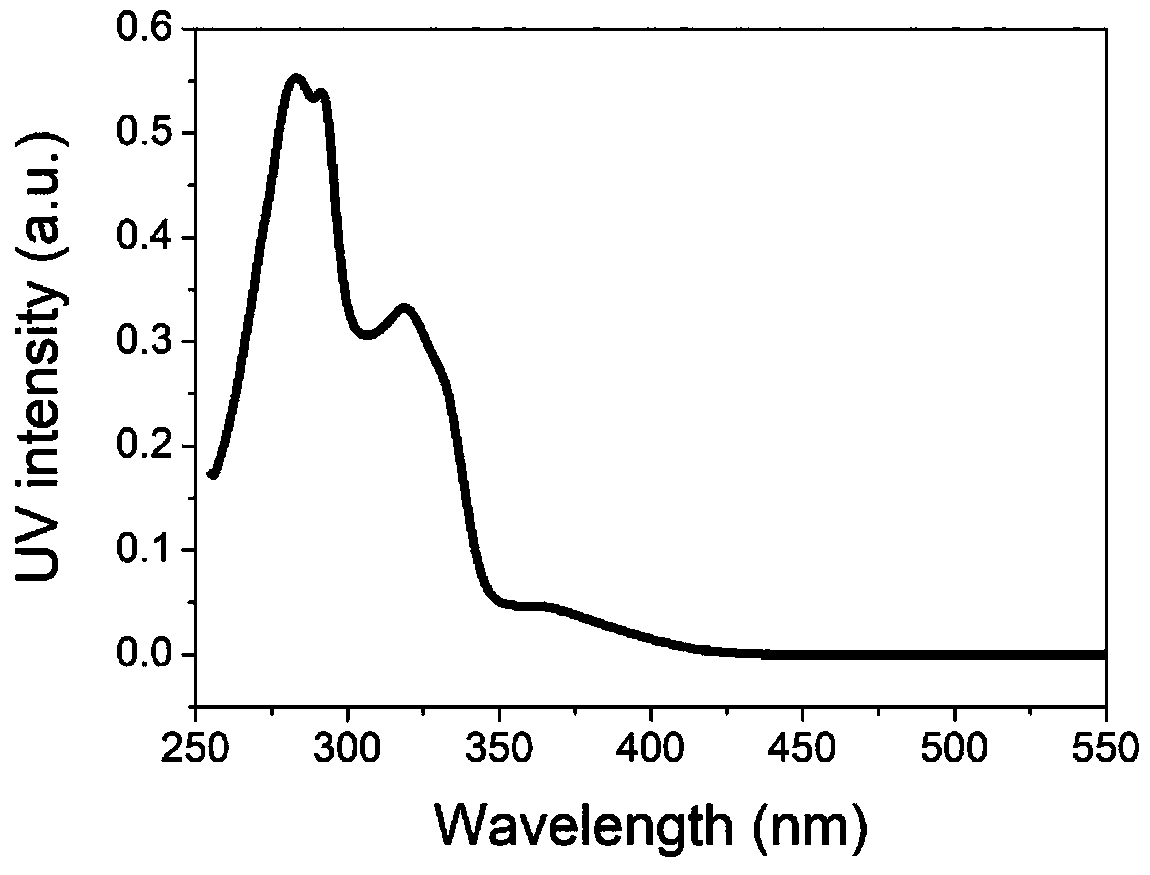
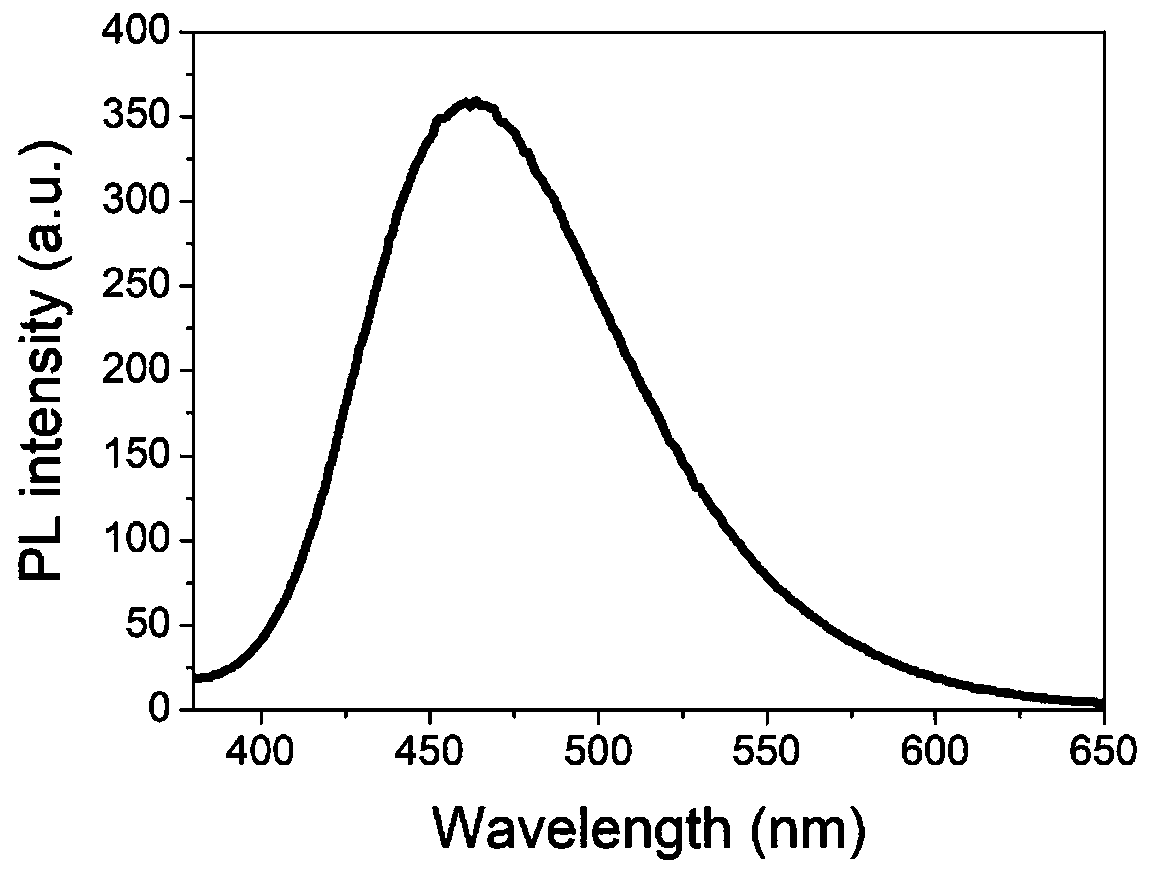
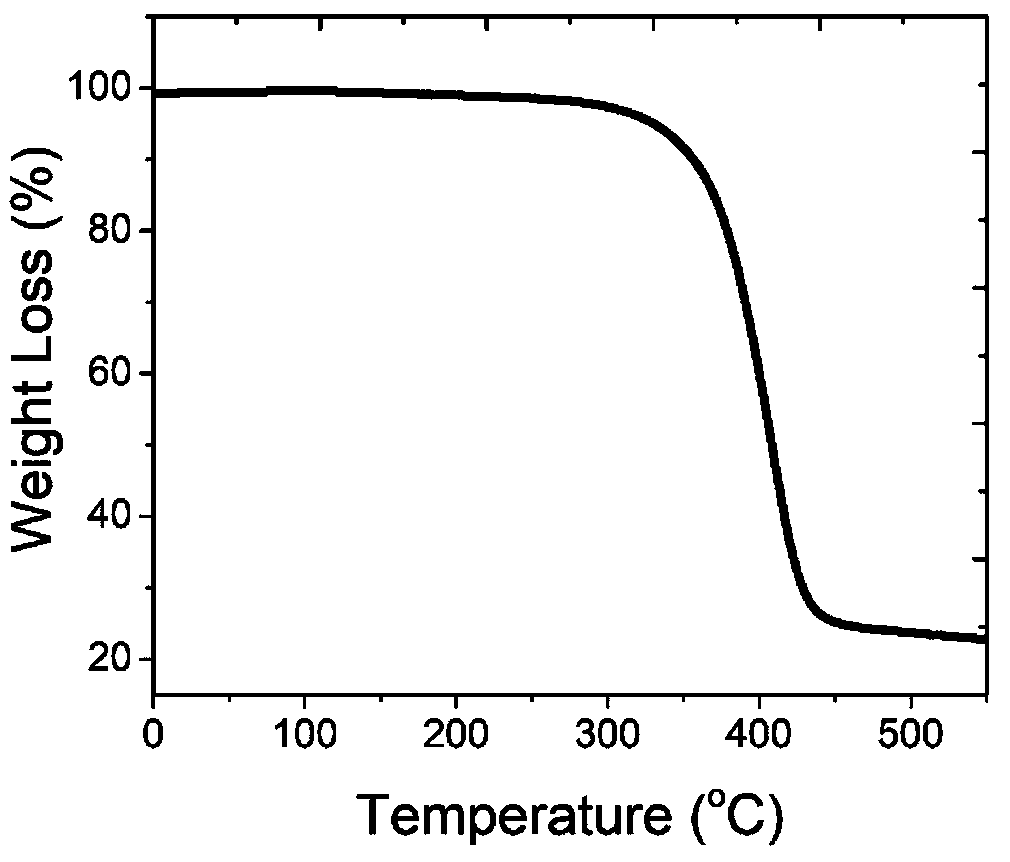
Preparation and application of thermally active delayed fluorescent material on basis of isoindigo unit
A delayed fluorescence, thermally active technology, applied in the manufacturing of luminescent materials, electrical components, semiconductor/solid-state devices, etc., can solve the problems of low efficiency of OLEDs, and achieve the effect of enhancing electron absorption ability, small energy level difference, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] Add 6,6'-dibromoisoindigo (1.0g, 2.4mmol), methyl iodide (0.7g, 5mmol), NaH (0.07mg, 3mmol) and DMF (20mL) successively in a 100mL single-necked bottle, and the mixture is Heat to 80oC and stir for 24 hours. After the reactant was cooled to room temperature, the mixture was extracted with CH2Cl2 (3×30mL); the organic layer was washed with water (60mL), dried, and evaporated under reduced pressure to remove the solvent; the residue was separated by column chromatography using petroleum ether as the eluent to obtain compound 1 0.8 g (yield: 75%).
Embodiment 2
[0021]
[0022] Add compound 1 (0.6g, 1.3mmol), malononitrile (0.3g, 4.0mmol), Al2O3 (0.1g, 1mmol) and toluene (20mL) successively in a 100mL single-necked bottle, and the mixture is heated to 85oC under nitrogen and stirred for 24 Hour. After the reactant was cooled to room temperature, the mixture was extracted with CH2Cl2 (3×30mL); the organic layer was successively washed with water (60mL), dried, and evaporated under reduced pressure to remove the solvent; the residue was washed with petroleum ether:dichloromethane (V:V=1: 1) The eluent was separated by column chromatography and then recrystallized to obtain 0.35 g of the target product (yield: 62%).
Embodiment 3
[0024]
[0025] Add compound 2 (0.4g, 0.74mmol), diphenylamine (0.4g, 2.2mmol), tetrakistriphenylphosphine palladium (187mg, 0.0162mmol), ethanol (10mL), toluene (30mL) and 2M aqueous potassium carbonate solution (10 mL), the mixture was heated to 90°C under nitrogen and stirred for 24 hours. After the reactant was cooled to room temperature, the mixture was extracted with CH2Cl2 (3×30mL); the organic layer was successively washed with water (60mL), dried, and evaporated under reduced pressure to remove the solvent; the residue was dichloromethane (V:V=1: 2) 300 mg of compound 3 was obtained by column chromatography as the eluent (yield: 60%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com