Stable sterilizing solution for clinic
A sterilization, solution technology, applied in the direction of chemicals, disinfectants, bactericides for biological control, etc., can solve the problems of increasing solution corrosion, skin corrosion, easy decomposition, etc., to reduce unstable factors and improve sterilization. Active, avoid serious pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
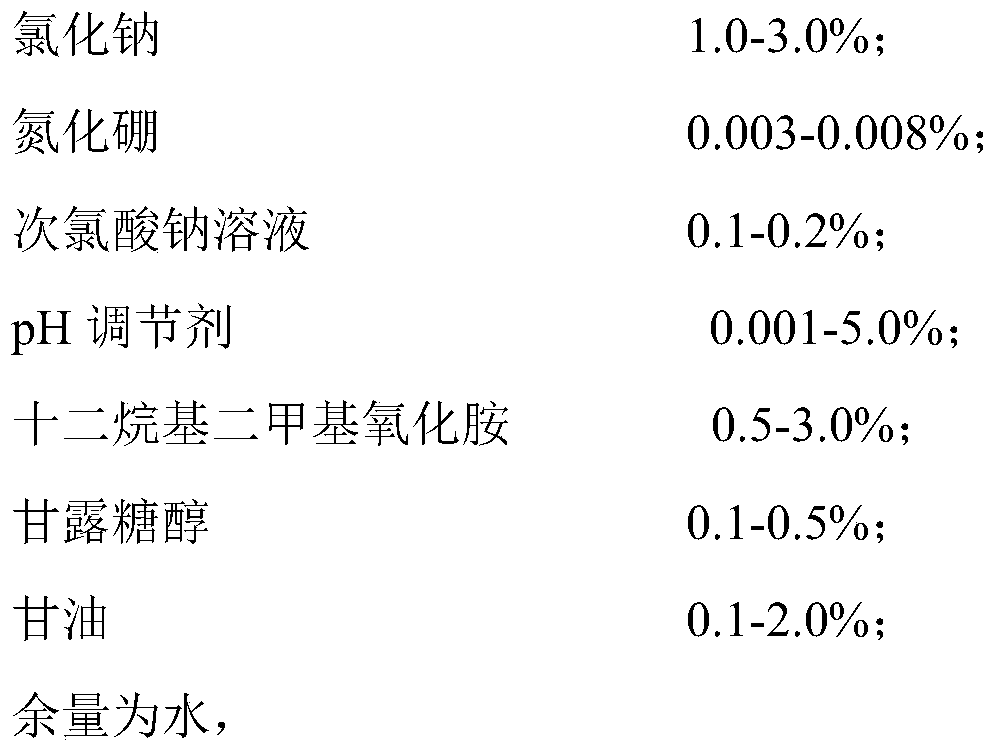
[0044] S1. Add 1Kg of sodium chloride, 3g of boron nitride and 100g of mannitol into 99.49L of water and stir evenly, then add 0.1L of food-grade sodium hypochlorite solution with an available chlorine concentration of 10%, and mix thoroughly to obtain solution A;
[0045] S2. Slowly add 0.5Kg of dodecyldimethylamine oxide and 0.1Kg of glycerin to solution A in turn, stir while adding, and mix well;
[0046] S3. Finally, slowly add 5M hydrochloric acid, monitor the pH value of the entire solution system in real time during the addition process, stop adding when the pH value of the entire solution system is 5.8-6.5, and stir for another 30 minutes;
[0047] S4. The final solution is tested for pH, available chlorine concentration, and clarity, and subpackaged and packaged after passing the test.
preparation Embodiment 2
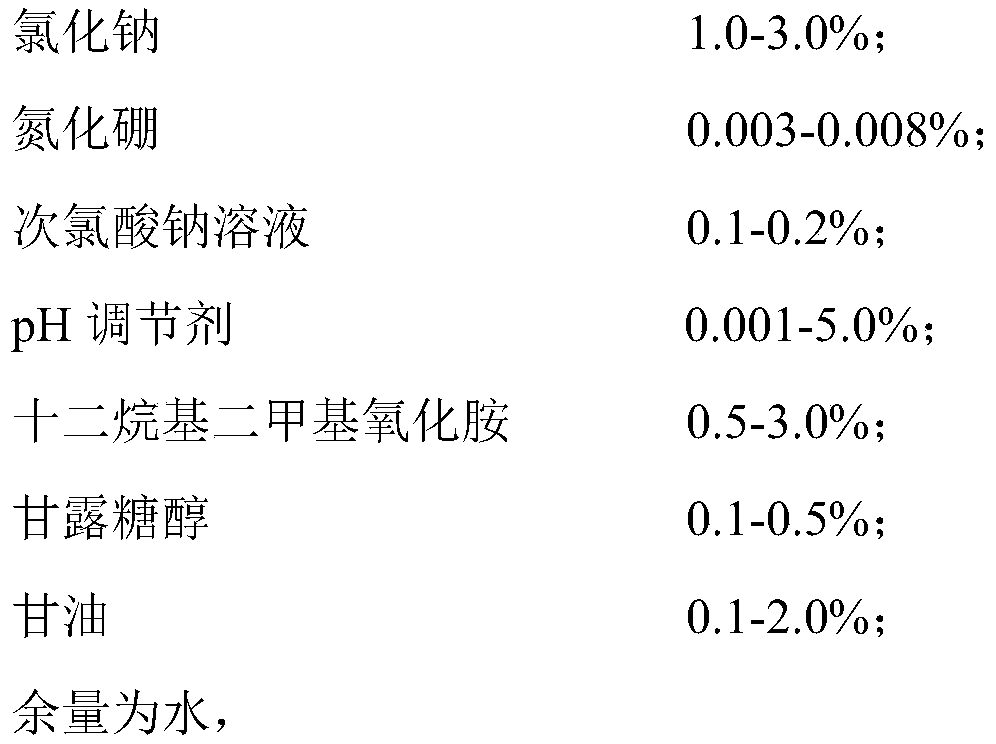
[0049] S1. Add 3Kg of sodium chloride, 8g of boron nitride and 500g of mannitol into 99.48L of water and stir evenly, then add 0.2L of food-grade sodium hypochlorite solution with an available chlorine concentration of 10%, and mix thoroughly to obtain solution A;
[0050] S2. Slowly add 3Kg of dodecyl dimethyl amine oxide and 2Kg of glycerin successively to solution A, stir while adding, and mix well;
[0051] S3. Finally, slowly add 5M hydrochloric acid, monitor the pH value of the entire solution system in real time during the addition process, stop adding when the pH value of the entire solution system is 5.8-6.5, and stir for another 30 minutes;
[0052] S4. The final solution is tested for pH, available chlorine concentration, and clarity, and subpackaged and packaged after passing the test.
preparation Embodiment 3
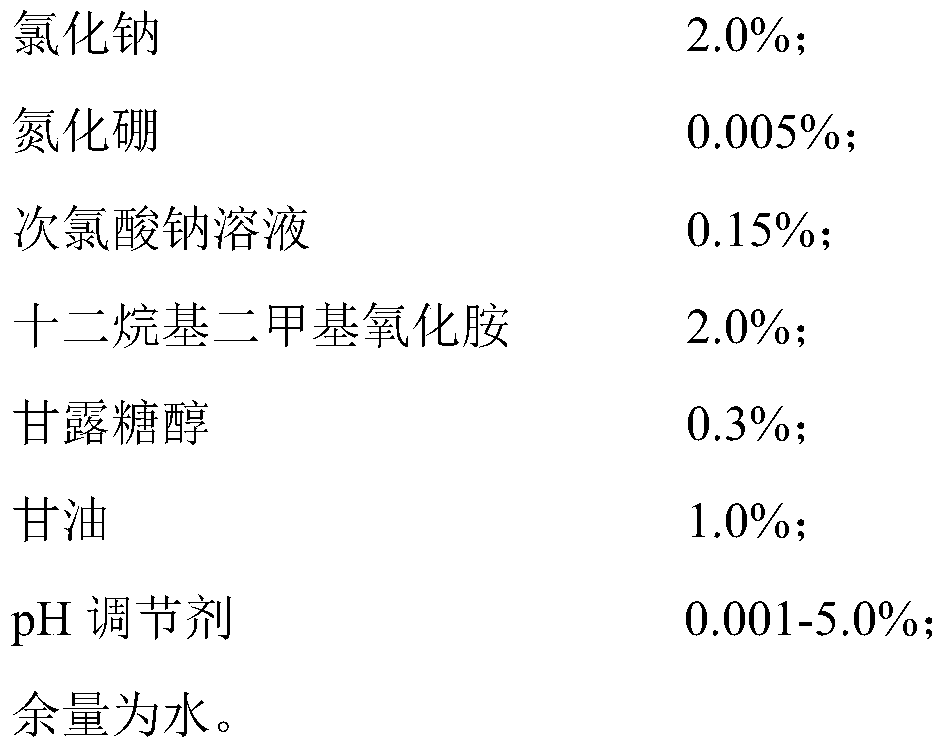
[0054] S1. Add 2Kg of sodium chloride, 5g of boron nitride and 300g of mannitol into 99.68L of water and stir evenly, then add 0.15L of food-grade sodium hypochlorite solution with an available chlorine concentration of 10%, and mix thoroughly to obtain solution A;
[0055] S2. Slowly add 2Kg of dodecyl dimethyl amine oxide and 1Kg of glycerin to solution A in turn, stir while adding, and mix well;
[0056] S3. Finally, slowly add 5M hydrochloric acid, monitor the pH value of the entire solution system in real time during the addition process, stop adding when the pH value of the entire solution system is 5.8-6.5, and stir for another 30 minutes;
[0057] S4. The final solution is tested for pH, available chlorine concentration, and clarity, and subpackaged and packaged after passing the test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com