Heavy metal removal preparation, and synthesis method and application thereof
A synthesis method and heavy metal technology, applied in chemical instruments and methods, other chemical processes, water/sludge/sewage treatment, etc., can solve problems such as secondary pollution, difficult post-treatment of regeneration fluid, and inability to remove, etc., to achieve a perfect connection , the effect of increasing the difficulty of synthesis and the cost of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
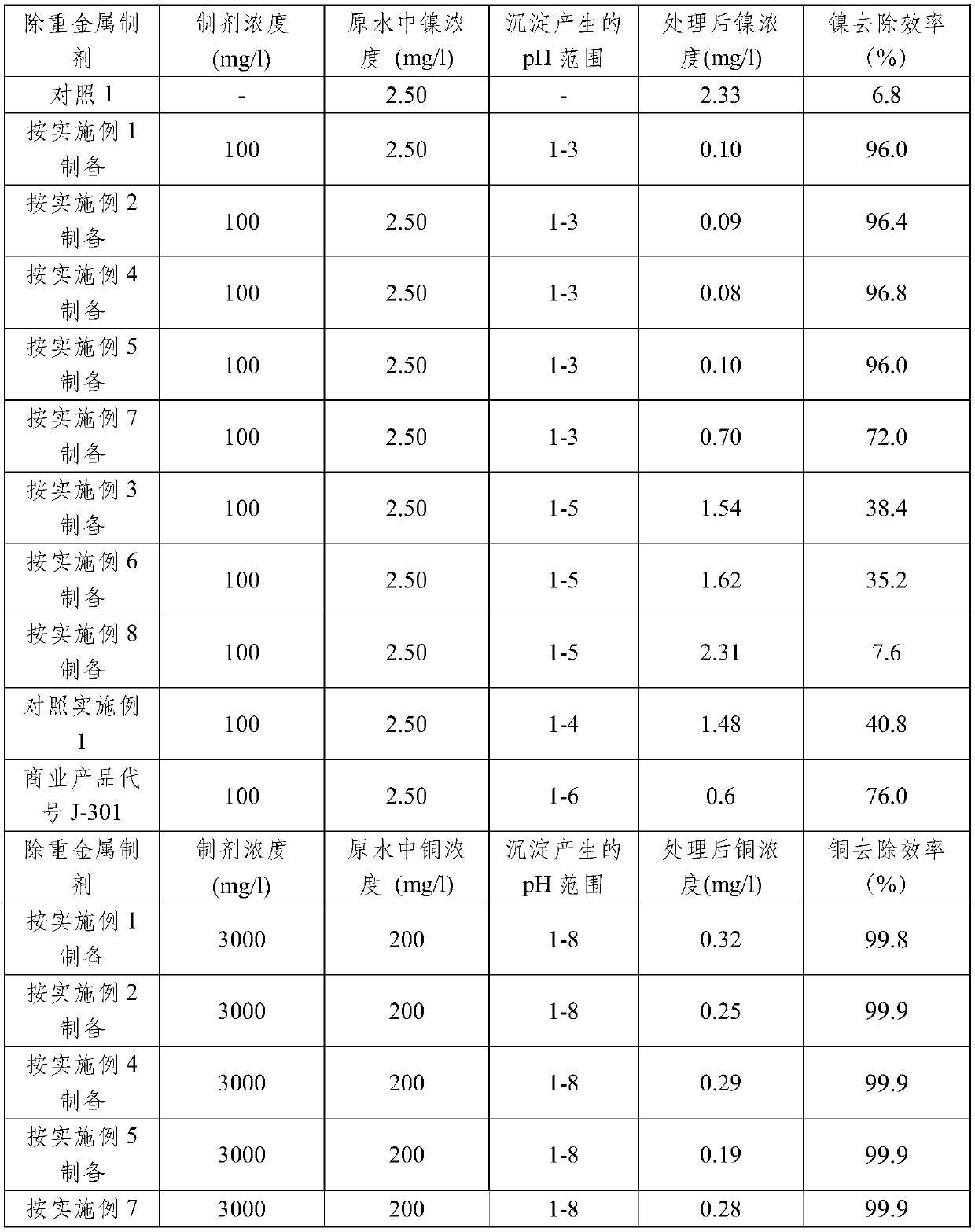
Embodiment 1
[0083] Add 6.20g of 1,2-dichloroethane and 8.50g of ammonia solution with a concentration of 25-28% (the molar ratio of dichloroethane / ammonia is about 1:2) into a 100ml reactor, seal it, and put it under magnetic force at 100°C React under stirring for 4h;
[0084] Slowly add 5ml of 40% sodium hydroxide aqueous solution in the reactor again, adjust pH to 9.6, then add 9.50g carbon disulfide (the mol ratio of dichloroethane / NaOH / carbon disulfide is about 1:0.8:2), seal, 50 ℃ under magnetic stirring for 4h.
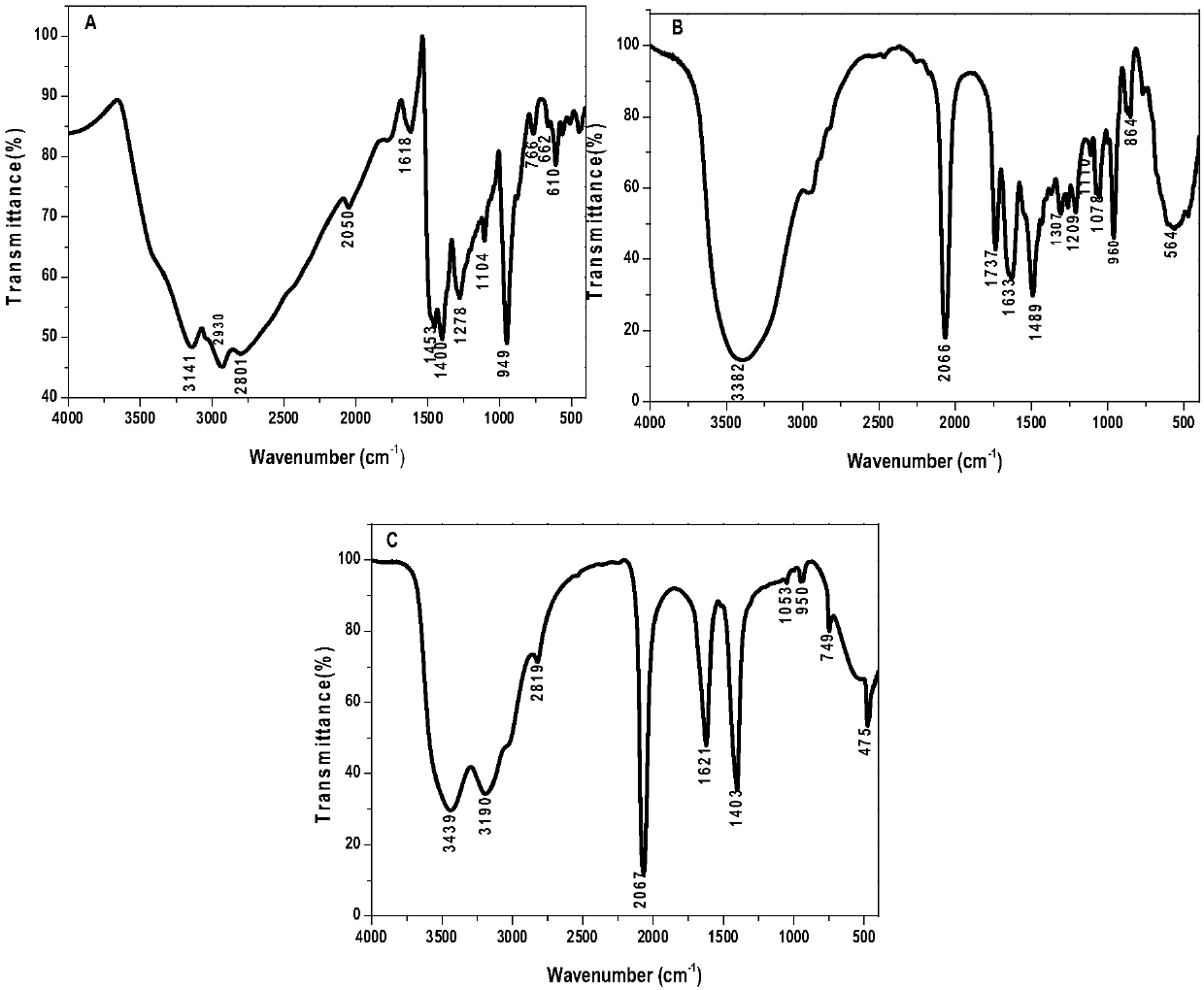
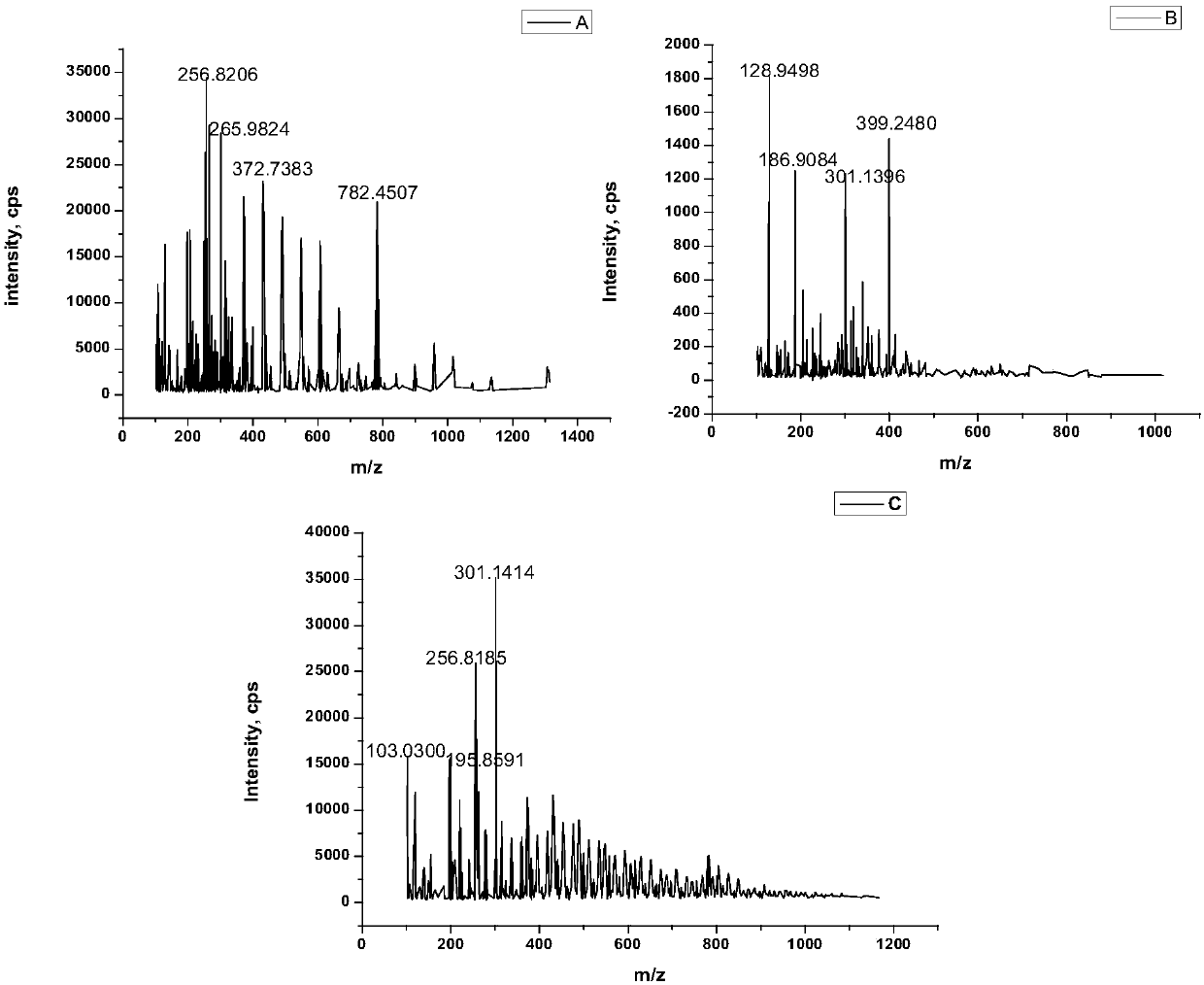
[0085] Cooling after the above-mentioned process ends, insoluble part (dithiocarbamic acids) in the product is dissolved in alkali again, obtains the heavy metal removal preparation mentioned in the present invention after remaining ammonia evaporates, and gained yield (dichloroethane's conversion) was 86%. Infrared spectrum (FT-IR) and high resolution mass spectrometry (TOF-MS) of the obtained preparation are as follows: figure 1 and figure 2 Shown in A.
Embodiment 2
[0087] Add 6.20g 1,2-ethylene dichloride, 8.50g concentration in the 100ml reactor and be the aqueous ammonia solution of 25-28%, 15ml concentration is the aqueous sodium carbonate solution of 40% (the mole of ethylene dichloride / ammonia / sodium carbonate The ratio is about 1:2:0.9), sealed, and reacted at 100°C for 4h;
[0088] Add 9.50 g of carbon disulfide to the reactor (the molar ratio of dichloroethane / carbon disulfide is about 1:2), seal it, and react for 4 hours under magnetic stirring at 60°C.
[0089] After the above process is finished, cool down, and dissolve the insoluble part (dithiocarbamic acids) in the product with alkali, and evaporate the remaining ammonia to obtain the heavy metal removal preparation mentioned in the present invention, with a yield of 83.7%. Infrared spectrum (FT-IR) and high resolution mass spectrometry (TOF-MS) of the obtained preparation are as follows: figure 1 and figure 2 Shown in B.
Embodiment 3
[0091] In the 100ml reactor, add 6.20g 1,2-dichloroethane, 8.5g concentration and be 25-28% ammonia solution, 5ml40% sodium hydroxide aqueous solution, 9.50g carbon disulfide (dichloroethane / ammonia / hydroxide The molar ratio of sodium / carbon disulfide is about 1:2:0.8:2), sealed, and reacted under magnetic stirring at 100°C for 4h. The reaction kettle was opened to take out the product, and all finished products were obtained after evaporation of the remaining ammonia, which was the heavy metal removal preparation mentioned in the present invention, and the yield was 89.3%. Infrared spectrum (FT-IR) and high resolution mass spectrometry (TOF-MS) of the obtained preparation are as follows: figure 1 and figure 2 Shown in C.
[0092] The remaining ammonia collected after evaporative cooling is recovered and used as a reactant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com