<18>F-labeled fluoropyridylformylglycine, and preparation method and application thereof
A technology of fluoropicolinoylglycine and picolinoylglycine, which is applied in the field of fluoropicolinoylglycine and its preparation, can solve the problems of long time, cumbersome synthesis steps, inability to obtain high-quality kidney images and the like, and achieves excellent stability, good biological performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
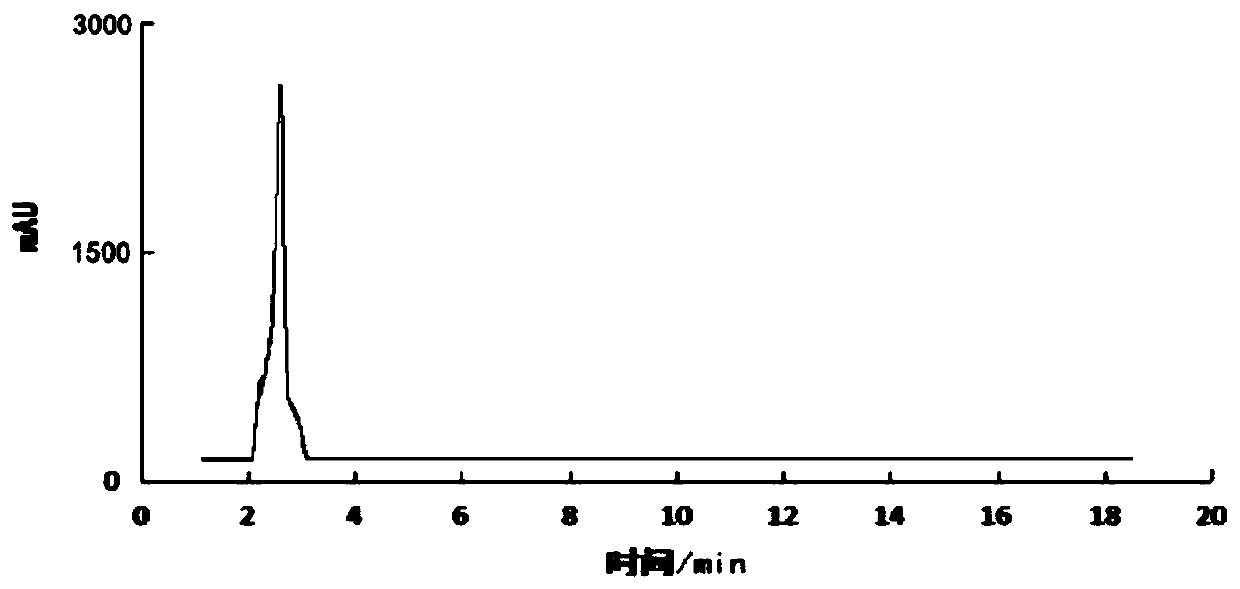
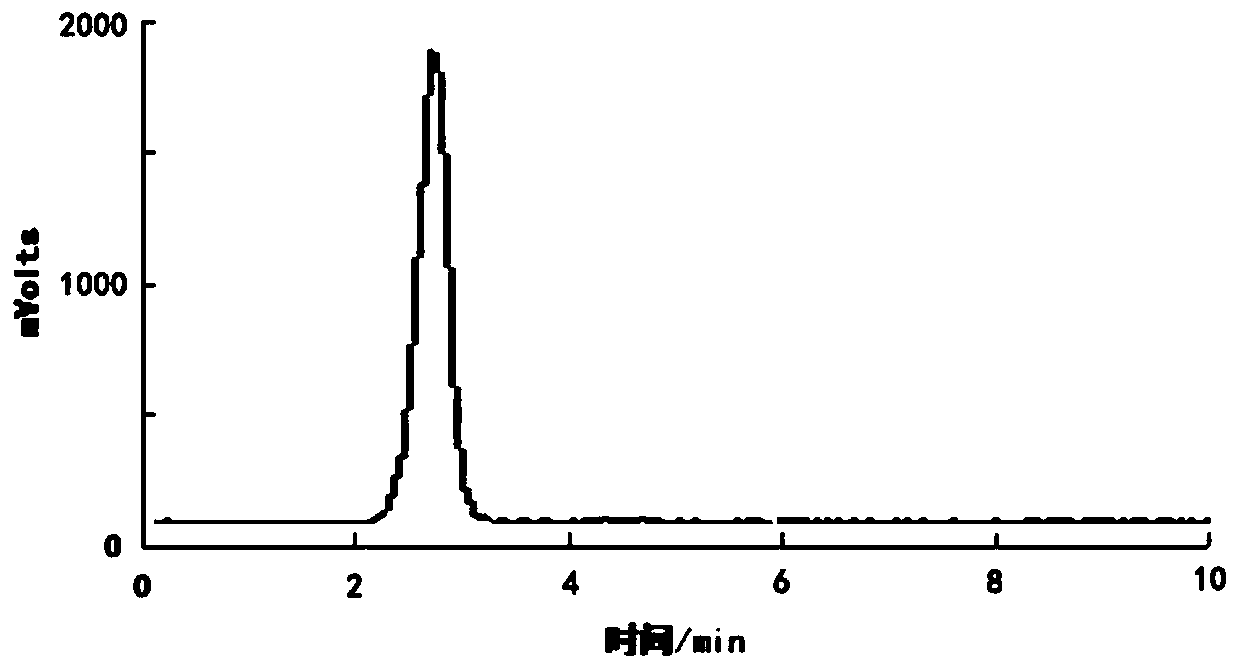
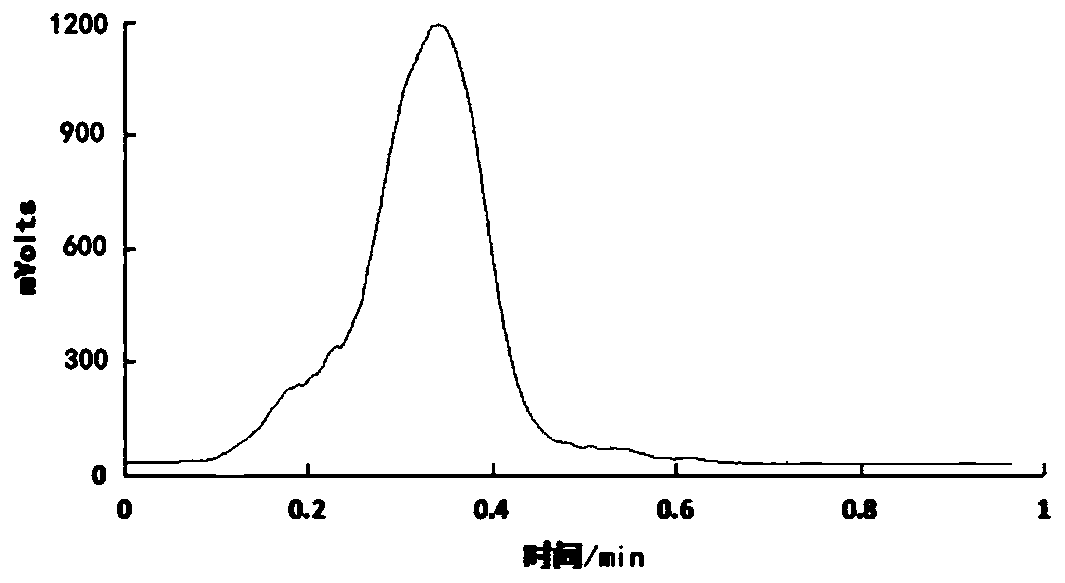
Image
Examples
preparation example Construction
[0027]The present invention also provides a kind of described in above-mentioned technical solution 18 The preparation method of the fluoropicolinylglycine of F mark, comprises the steps:
[0028] (1) bromopicolinylglycinate, solvent and [ 18 F] - The source is mixed, the nucleophilic reaction is carried out, and after purification, the 18 F-fluoropicolinyl glycinate; the [ 18 F] - source contains [ 18 F] - And catalyst; The bromopicolyl glycinate is 6-bromo-3-pyridineformyl glycinate, 5-bromo-2-pyridineformyl glycinate or 4-bromo-2-pyridineformyl glycinate;
[0029] (2) the above 18 F-fluoropicolinylglycinate is mixed with sodium hydroxide solution for hydrolysis reaction, then passed through an acidic ion exchange column, and then filtered through a sterile filter membrane to obtain 18 F-labeled fluoropicolinylglycine.
[0030] The raw materials used in the preparation method provided by the invention are easy to obtain, and the obtained 18 F-labeled fluoropicoliny...
Embodiment 1
[0075] 6-bromo-3-pyridinecarboxylic acid (1.00g, 4.95mmol), N,N-dicyclohexylcarbodiimide (1.22g, 5.94mmol), 4-dimethylaminopyridine (0.36g, 2.97mmol) and Anhydrous dichloromethane (20 mL) was mixed, stirred evenly in an ice-water bath (0° C.), and reacted for 30 minutes to obtain a reaction solution containing activated 6-bromo-3-pyridinecarboxylic acid;
[0076] Triethylamine (0.82mL, 5.94mmol), glycine methyl ester hydrochloride (0.75g, 5.94mmol) and anhydrous dichloromethane (15mL) were mixed and added dropwise to the activated 6-bromo-3-pyridine In the reaction solution of formic acid, after the dropwise addition, return to room temperature (23°C), continue to stir the reaction for 20h, TLC detects that the reaction is complete, then filter, and the obtained filtrate is subjected to vacuum rotary evaporation, and then the 300-400 mesh silica gel column is used as The chromatographic column is subjected to column chromatography, and the eluent used in the column chromatogra...
Embodiment 2
[0084] According to the method of Example 1, the raw material 6-bromo-3-pyridinecarboxylic acid was replaced by 5-bromo-2-pyridinecarboxylic acid to prepare 5-[ 18 F]-fluoro-2-pyridinecarbonylglycine.
[0085] According to the method test of embodiment 1 5-[ 18 The radiochemical yield after correction of F]-fluoro-2-pyridineformylglycine was 10±3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com