Silane coupling agent with double stimulation response and preparation method and application thereof
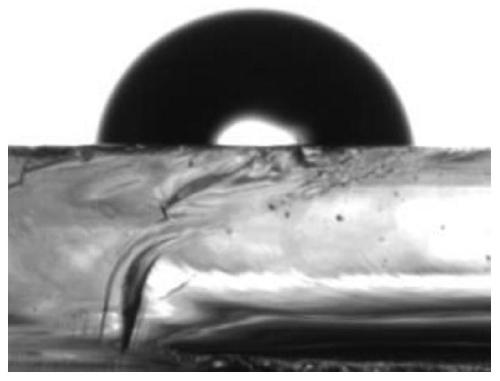
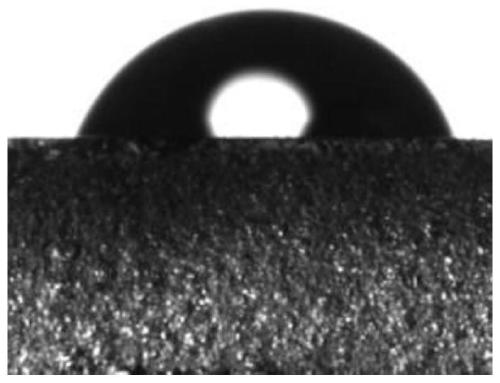
A technology of dual stimulus response and silane coupling agent, applied in the field of preparation of silane coupling agent, can solve the problems of cumbersome conditions, complex synthesis, single control method, etc., and achieve the effect of simple modification method and improved hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
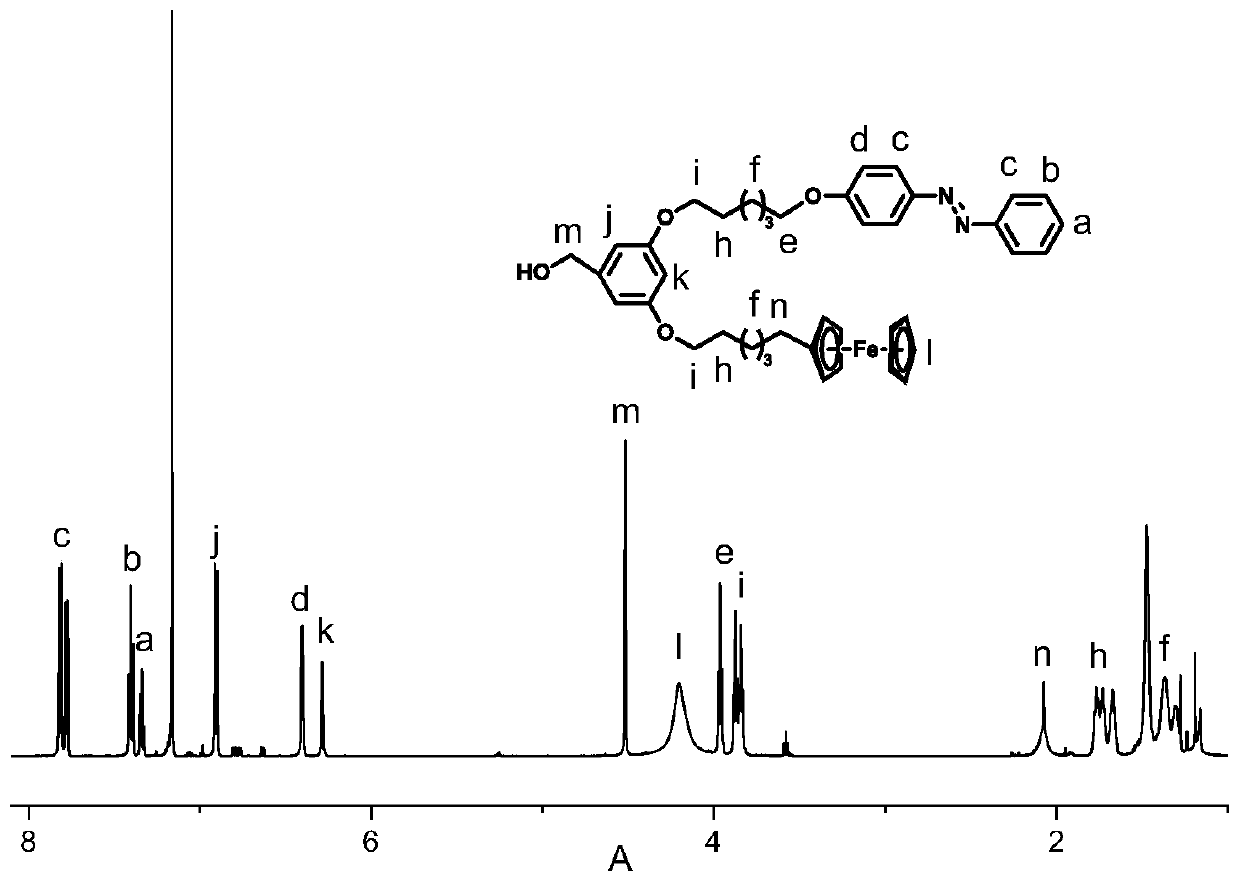
[0044] Under nitrogen protection, add 1g (2.8mmol) 3-hydroxyl, 5-ferrocenehexyloxymethylbenzoate, 1.52g (4.2mmol) hexabromohexyloxyazobenzene, 1.37g to a 150mL three-necked flask (4.2mmol) cesium carbonate, 50mL N, N-dimethylformamide (DMF), warming up to 85 ℃ for reaction, during the reaction, use thin-layer chromatography to determine the reaction process, after 12 hours, the reaction is complete, stop heating, and cool After reaching room temperature, the cesium carbonate was removed by suction filtration, the cesium salt was rinsed with dichloromethane until it was colorless, and the solvent was removed by rotary evaporation under reduced pressure to obtain an orange-yellow crude product. The crude product was dissolved in methyl ethyl ketone, put into the refrigerator to cool and crystallize, and repeated 3 times to obtain 3-ferrocenehexyloxy, 5-azophenylhexyloxymethyl benzoate.
[0045]Under nitrogen protection, add 10mL of anhydrous tetrahydrofuran to a 150mL three-neck...
Embodiment 2
[0055] Under nitrogen protection, add 1g (2.6mmol) 3-hydroxyl, 5-ferrocene octyloxymethyl benzoate, 0.8g (2.6mmol) hexabromooctyloxyazobenzene, 0.54g to a 150mL three-necked flask (3.9mmol) Potassium Carbonate, 50mL N, N-dimethylformamide (DMF), warming up to 85 ° C for reaction, during the reaction, use thin layer chromatography to determine the reaction process, after 12 hours, the reaction is complete, stop heating, and cool After reaching room temperature, potassium carbonate was removed by suction filtration, the potassium salt was rinsed with dichloromethane until it was colorless, and the solvent was removed by rotary evaporation under reduced pressure to obtain an orange-yellow crude product. The crude product was dissolved in methyl ethyl ketone, put into the refrigerator to cool and crystallize, and repeated 3 times to obtain 3-ferrocenyl octyloxy, 5-azobenzyloxybenzoic acid methyl ester.
[0056] Under nitrogen protection, 10 mL of anhydrous tetrahydrofuran was adde...
Embodiment 3
[0062] Under nitrogen protection, add 1g (2.5mmol) 3-hydroxy, 5-ferrocenedecyloxymethylbenzoate, 1.54g (3.75mmol) hexabromodecyloxyazobenzene, 1.22g to a 150mL three-necked flask (3.75mmol) cesium carbonate, 50mL N, N-dimethylformamide (DMF), warming up to 85 ℃ for reaction, during the reaction, use thin layer chromatography to determine the reaction process, after 12 hours, the reaction is complete, stop heating, and cool After reaching room temperature, the cesium carbonate was removed by suction filtration, the cesium salt was rinsed with dichloromethane until it was colorless, and the solvent was removed by rotary evaporation under reduced pressure to obtain an orange-yellow crude product. Dissolve the crude product in butanone, put it in the refrigerator to cool and crystallize, and repeat 3 times to obtain 3-ferrocenyldecyloxy, 5-azophenyldecyloxybenzoic acid methyl ester.
[0063] Under nitrogen protection, 10 mL of anhydrous tetrahydrofuran was added to a 150 mL three-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com