A kind of silane coupling agent with double stimulation response and its preparation method and application
A dual-stimuli-response, silane coupling agent technology, applied in the field of silane coupling agent preparation, can solve the problems of cumbersome conditions, complex synthesis, single control method, etc., and achieve the effect of improving hydrophobicity and simple modification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
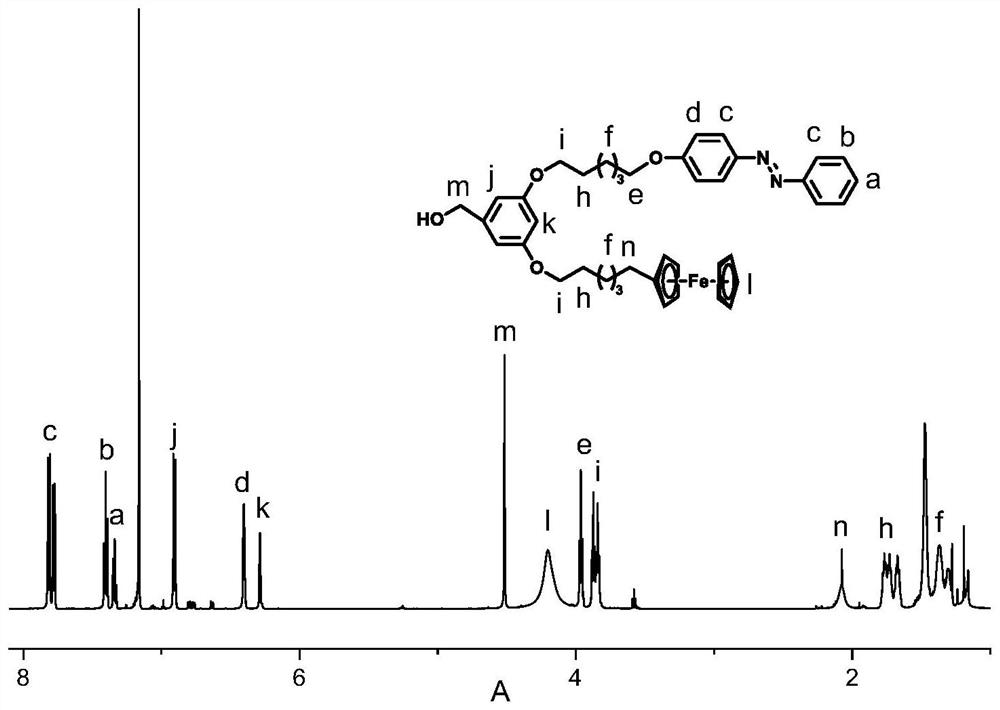
[0044] Under nitrogen protection, add 1g (2.8mmol) 3-hydroxyl, 5-ferrocenehexyloxymethylbenzoate, 1.52g (4.2mmol) hexabromohexyloxyazobenzene, 1.37g to a 150mL three-necked flask (4.2mmol) cesium carbonate, 50mL N, N-dimethylformamide (DMF), warming up to 85 ℃ for reaction, during the reaction, use thin-layer chromatography to determine the reaction process, after 12 hours, the reaction is complete, stop heating, and cool After reaching room temperature, the cesium carbonate was removed by suction filtration, the cesium salt was rinsed with dichloromethane until it was colorless, and the solvent was removed by rotary evaporation under reduced pressure to obtain an orange-yellow crude product. The crude product was dissolved in butanone, placed in a refrigerator to cool and crystallize, and repeated three times to obtain methyl 3-ferrocenehexyloxy-5-azophenylhexyloxybenzoate.
[0045]Under nitrogen protection, add 10mL of anhydrous tetrahydrofuran to a 150mL three-necked flask,...
Embodiment 2
[0055] Under nitrogen protection, add 1g (2.6mmol) 3-hydroxyl, 5-ferrocene octyloxymethyl benzoate, 0.8g (2.6mmol) hexabromooctyloxyazobenzene, 0.54g to a 150mL three-necked flask (3.9mmol) Potassium Carbonate, 50mL N, N-dimethylformamide (DMF), warming up to 85 ° C for reaction, during the reaction, use thin layer chromatography to determine the reaction process, after 12 hours, the reaction is complete, stop heating, and cool After reaching room temperature, potassium carbonate was removed by suction filtration, the potassium salt was rinsed with dichloromethane until it was colorless, and the solvent was removed by rotary evaporation under reduced pressure to obtain an orange-yellow crude product. The crude product was dissolved in methyl ethyl ketone, put into the refrigerator to cool and crystallize, and repeated 3 times to obtain 3-ferrocenyl octyloxy, 5-azobenzyloxybenzoic acid methyl ester.
[0056] Under nitrogen protection, 10 mL of anhydrous tetrahydrofuran was adde...
Embodiment 3
[0062] Under nitrogen protection, in a 150mL three-necked flask, add 1g (2.5mmol) 3-hydroxy-5-ferrocenedecyloxymethyl benzoate, 1.54g (3.75mmol) hexabromodecyloxyazobenzene, 1.22g (3.75mmol) cesium carbonate, 50mL N, N-dimethylformamide (DMF), warming up to 85 ℃ for reaction, during the reaction, use thin layer chromatography to determine the reaction process, after 12 hours, the reaction is complete, stop heating, and cool After reaching room temperature, the cesium carbonate was removed by suction filtration, the cesium salt was rinsed with dichloromethane until it was colorless, and the solvent was removed by rotary evaporation under reduced pressure to obtain an orange-yellow crude product. The crude product was dissolved in butanone, placed in a refrigerator for cooling and crystallization, and repeated 3 times to obtain methyl 3-ferrocenedecyloxy-5-azobendecyloxybenzoate.
[0063] Under nitrogen protection, 10 mL of anhydrous tetrahydrofuran was added to a 150 mL three-n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com