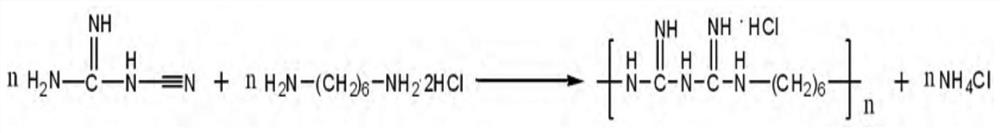
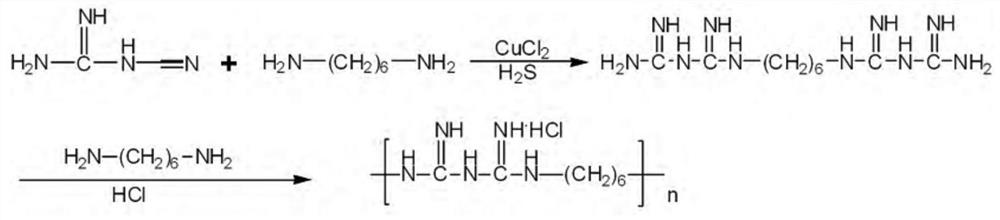
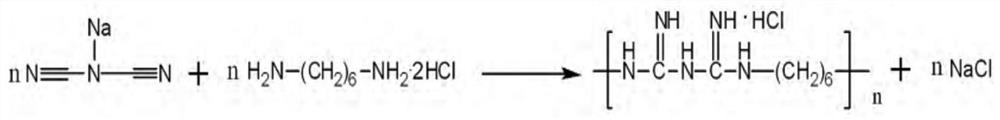Synthetic technology of polyhexamethylene biguanide hydrochloride
A technology of polyhexamethylene biguanide hydrochloride and synthesis process, which is applied to the addition of paper forming aids, the addition of non-polymeric organic compounds, and the addition of anti-biological reagents, etc., can solve the problem of high viscosity of the reaction system and easy burning of the stirring motor , low conversion rate of raw materials, etc., to achieve the effect of large degree of product polymerization, excellent broad-spectrum antibacterial activity, high molecular weight and cationic degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The synthetic technique of polyhexamethylene biguanide hydrochloride comprises the following steps:
[0050] (1) 1,6-dicyanoguanidine hydrochloride and hexamethylenediamine hydrochloride are dissolved in an organic solvent, and an addition reaction occurs at 100° C., the 1,6-biscyanoguanidine and The mol ratio of described hexamethylenediamine hydrochloride is 1: 1.05, and described organic solvent is diethylene glycol, and the consumption of described organic solvent is 3 times of 1,6-dicyanoguanidine hexane mass, so Described catalyst is 2-bromo-2-methylisobutylsiloxysilsesquioxane, and the consumption of described catalyst is 0.5 times of 1,6-dicyanoguanidine mass;
[0051] (2) After the addition reaction occurs, polyhexamethylene biguanide hydrochloride is obtained by performing self-condensation reaction at 150-160° C. under the action of a catalyst.
[0052] The polyhexamethylene biguanide hydrochloride synthesized in this example has confirmed the correctness of...
Embodiment 2
[0055] () Dissolve 1,6-dicyanoguanidine hydrochloride and hexamethylenediamine hydrochloride in an organic solvent, and an addition reaction occurs at 120° C., the 1,6-dicyanoguanidine and the The mol ratio of described hexamethylenediamine hydrochloride is 1: 1.07, and described organic solvent is diethylene glycol, and the consumption of described organic solvent is 4 times of 1,6-dicyanoguanidine hexane quality, and described The catalyst is azodiisopropylimidazoline hydrochloride, and the amount of the catalyst is 1 times the quality of 1,6-dicyanoguanidine;
[0056] (2) After the addition reaction occurs, polyhexamethylene biguanide hydrochloride is obtained by performing self-condensation reaction at 150-160° C. under the action of a catalyst.
[0057] The polyhexamethylene biguanide hydrochloride synthesized in this example has confirmed the correctness of the target product and the quality of the product through NMR and high-performance liquid chromatography detection ...
Embodiment 3
[0060] (1) Dissolve 1,6-dicyanoguanidine hexane and hexamethylenediamine hydrochloride in an organic solvent, and an addition reaction occurs at 130° C., the 1.6-dicyanoguanidine hexane and the The mol ratio of hexamethylenediamine hydrochloride is 1: 1.1, and described organic solvent is diethylene glycol, and the consumption of described organic solvent is 6 times of 1,6-dicyanoguanidine hexane quality, and described catalyst It is 2-bromo-2-methylisobutylsiloxysilsesquioxane, and the amount of the catalyst is 1.5 times the mass of 1,6-dicyanoguanidinohexane;
[0061] (2) After the addition reaction occurs, polyhexamethylene biguanide hydrochloride is obtained by performing self-condensation reaction at 150-160° C. under the action of a catalyst.
[0062] The polyhexamethylene biguanide hydrochloride synthesized in this example has confirmed the correctness of the target product and the quality of the product through NMR and high-performance liquid chromatography detection t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com