Nuclear medicine of structure modified RGD polypeptide
A drug and peptide technology, applied in the field of nuclear medicine drugs, can solve the problems of complex synthesis, high cost, and low tumor uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 168G
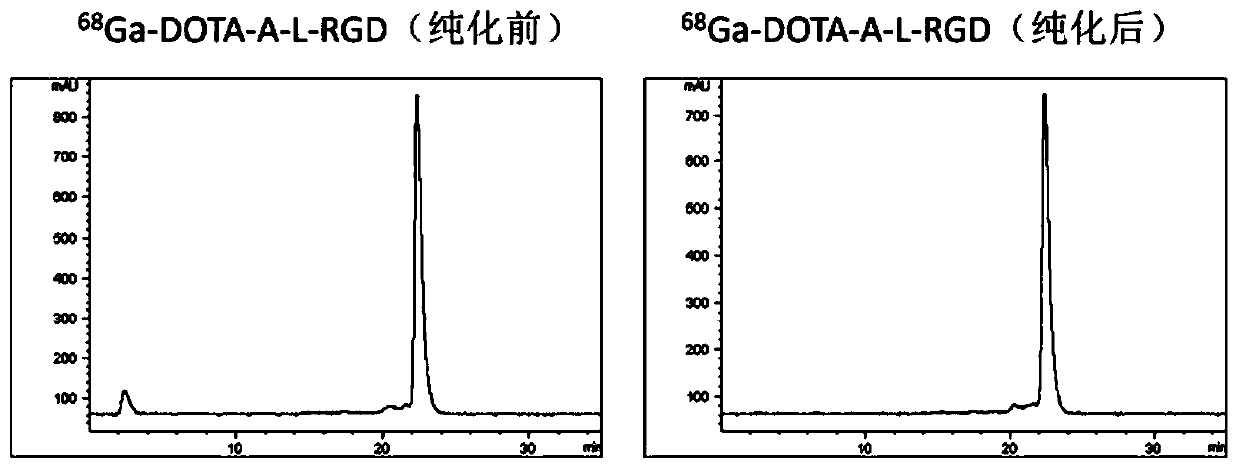
[0081] Example 1 68 Preparation of Ga-DOTA-A-c(RGDfk)
[0082] The synthetic route of DOTA-A-c (RGDfk) is as follows:
[0083]
[0084] (1) Synthesis of compound 1
[0085] Weigh 4-(4-iodophenyl)butanoic acid (9.8 mg, 33.8 mmol) into a one-necked bottle, dissolve it in 400 μL DMF, and add NHS (3.9 mg, 33.9 mmol). Then add 5.3 μL of DIC. The reaction was stirred at 30°C for 2 hours, and monitored by TLC (ethyl acetate:petroleum ether:acetic acid=100:200:2) until the raw materials disappeared. After the reaction was completed, the reaction solution was dissolved in ethyl acetate, washed with water three times, the ethyl acetate phase was dried over anhydrous sodium sulfate, concentrated and separated by column chromatography (ethyl acetate:petroleum ether:acetic acid=100:200:2) , collected fractions for detection, and spin-dried the collected product to obtain 10.2 mg of a white solid, with a yield of 78%. The expected product was confirmed by MALDI-TOF mass spectrometry...
Embodiment 2
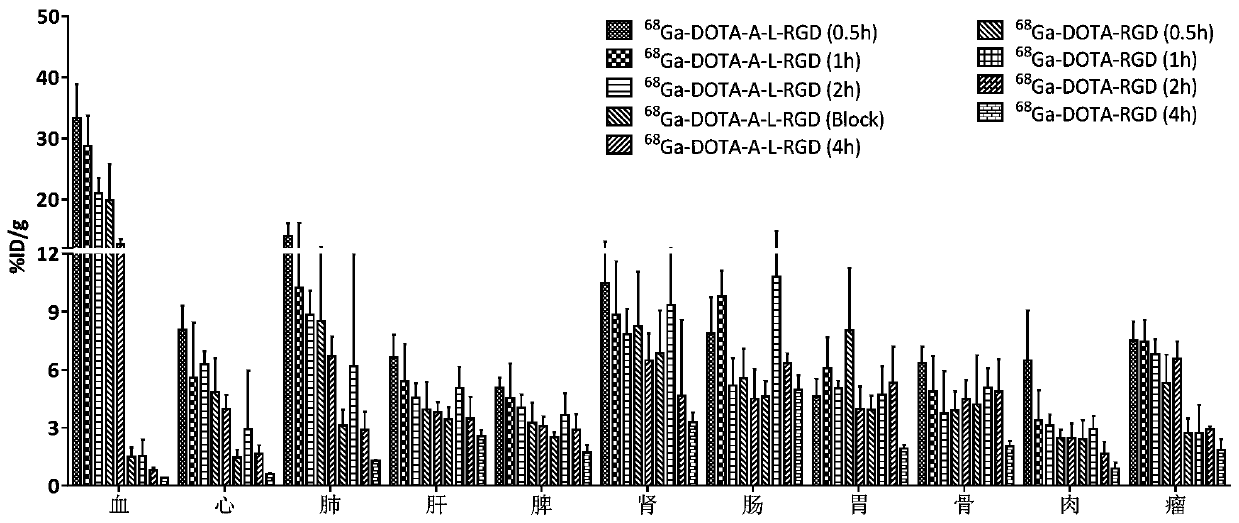
[0107] Example 2: In vivo biodistribution experiment of the molecular probe of Example 1
[0108] Thirty-six tumor-bearing C57BL / 6J mice were randomly divided into 9 groups with 4 mice in each group. The four groups were each injected with 0.1 mL of tail vein 68 Ga-DOTA-A-c (RGDfk) (about 1.85MBq), four groups of tail vein injection 68 Ga-DOTA-c (RGDfk) (about 1.85MBq) was sacrificed after blood was drawn at 0.5h, 1h, 2h, and 4h, and the heart, liver, spleen, lung, kidney, intestine, stomach, bone, meat, and tumor were dissected. . Measure mass and count radioactivity, weigh and measure radioactive cpm counts, and calculate percent injected dose per gram of tissue (%ID / g) after decay correction. Simultaneously inject 0.1 mL into the remaining group of tail veins 68 Ga-DOTA-A-c(RGDfk) and 0.05mL c(RGDfk) solution (0.5mg) were sacrificed one hour later, the organs were weighed and radioactive cpm counts were measured, and the percent injected dose per gram of tissue was calc...
Embodiment 3
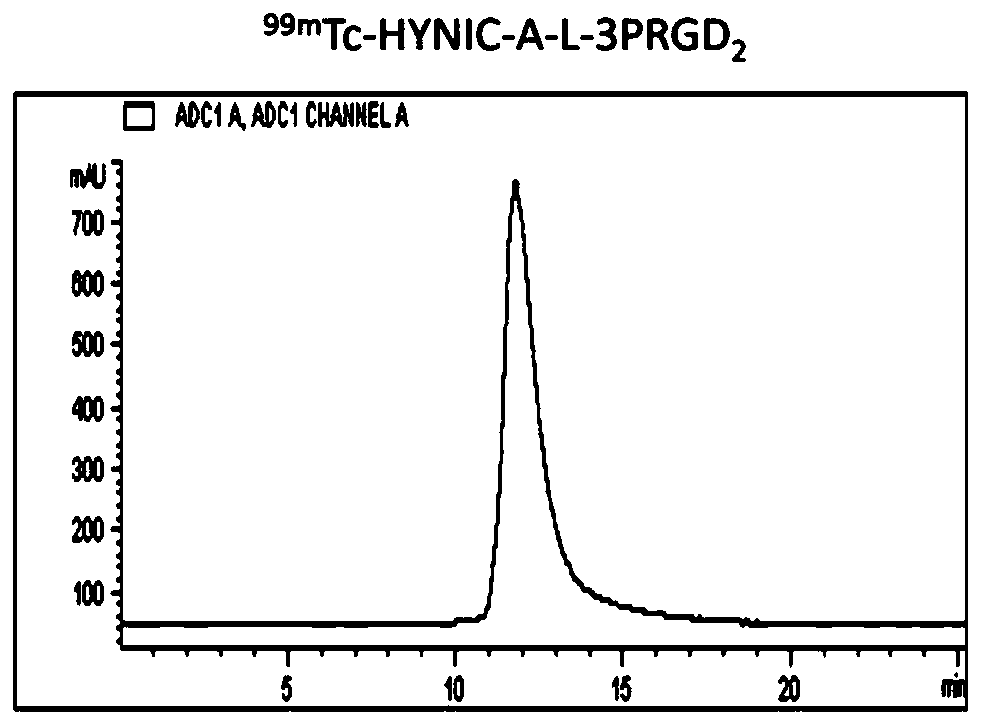
[0110] Example 3: 99m Tc-HYNIC-A-3PRGD 2 preparation of
[0111] HYNIC-A-3PRGD 2 The schematic diagram of the synthetic route is as follows:
[0112]
[0113] R=3PRGD 2
[0114] (1) Synthesis of Compounds 1, 2, and 3
[0115] The synthesis method of the compound refers to Example 1.
[0116] (2) Synthesis of compound 7
[0117] Weigh compound 3 (5.64mg, 7.65mmol) in 1mL EP tube, dissolve in 500μL DMF, add 3PRGD 2 (15.75mg, 7.8mmol), then add DIEA to adjust pH=8.5, and react overnight at room temperature. The reaction was monitored using HPLC. Using HPLC (High Performance Liquid Chromatography) Method 1, the product peaked at 13.17 min. Collected by HPLC and identified by mass spectrometry, it was determined to be the expected product.
[0118] MALDI-TOF-MS: m / z=2680.89 (chemical formula: C 123 h 181 IN 24 o 35 , Calculated molecular weight: 2681.22Da.).
[0119] (3) Synthesis of Compound 8
[0120] Add 125 μL of piperidine to the EP tube of the previous rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com