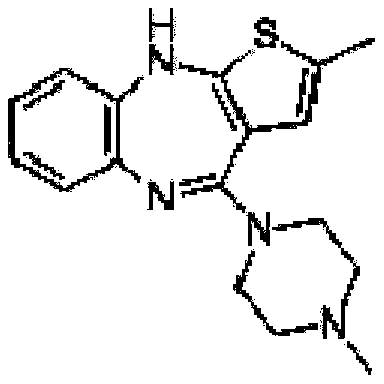
Olanzapine oral instant film and preparation method thereof
A technology of instant film and olanzapine, which is applied in the field of medicine, can solve the problems of high equipment requirements, difficult process, difficult dispersion, etc., and achieve the effect of simple preparation process, simple process flow and uniform drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
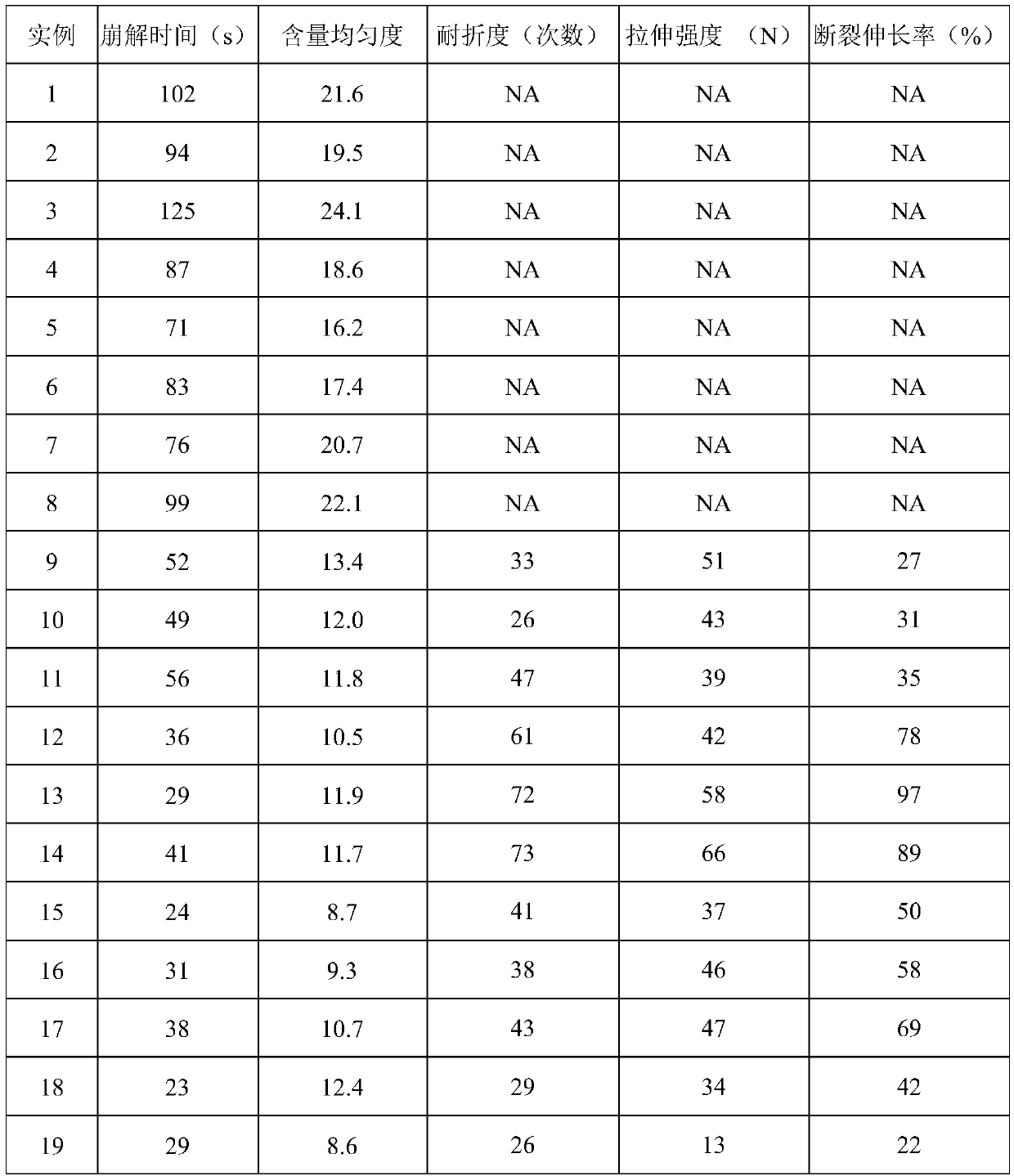
Examples
Embodiment 1
[0026] Add 2g of hypromellose to 10mL of distilled water, make it fully swell under constant temperature magnetic stirring at 75°C, then dissolve it in an ice bath, add 0.5g of olanzapine and 0.072g of oxalic acid (molar ratio 1:0.5) Add 0.4g of propylene glycol and 0.04g of sucralose to the film-forming material glue solution at the same time, stir at room temperature for 2 hours to obtain a uniform solution, apply it on a glass plate after ultrasonic degassing, dry at 50°C for 1 hour, and cut to obtain .
Embodiment 2
[0028] Add 2g of hypromellose to 10mL of distilled water, make it fully swell under constant temperature magnetic stirring at 75°C, and then dissolve it in an ice bath, mix 0.5g of olanzapine with 0.095g of succinic acid (molar ratio 1:0.5) Add it to the film-forming material glue, add 0.4g of propylene glycol and 0.04g of sucralose at the same time, stir at room temperature for 2 hours to obtain a uniform solution, apply it on a glass plate after ultrasonic degassing, dry at 50°C for 1 hour, and cut it. have to.
Embodiment 3
[0030] Add 2g of hypromellose to 10mL of distilled water, swell it fully under constant temperature magnetic stirring at 75°C, and then dissolve it in an ice bath, mix 0.5g of olanzapine with 0.093g of fumaric acid (molar ratio 1:0.5 ) into the film-forming material glue solution, simultaneously add 0.4 g of propylene glycol and 0.04 g of sucralose, stir at room temperature for 2 h to obtain a uniform solution, apply it on a glass plate after ultrasonic degassing, dry at 50 ° C for 1 h, and cut Instantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com