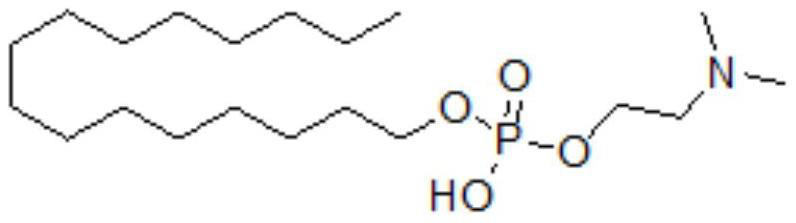
A kind of preparation method of miltefosine
A technology of miltefosine and cetyl alcohol, which is applied in the field of preparation of miltefosine, can solve the problems of large amount of dimethyl sulfate, difficulty in purification, limited application, etc., and achieves cheap and easy raw materials Obtaining and benefiting the effect of industrial scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] S1: Stir and dissolve 24.2g of n-hexadecanol (0.1mol) in 100mL of tetrahydrofuran, then add 11.2g of triethylamine (0.11mol), continue to stir well, and dissolve 18.4g of phosphorus oxychloride (0.12mol) Add it to 10mL of tetrahydrofuran, then lower the temperature to below 15°C, and slowly add it dropwise to the reaction system, controlling the temperature not to exceed 20°C. After the drop is complete, stir and react at 20°C for 2 hours, and the raw materials basically disappear under TLC monitoring. Then 10.68g of dimethylethanolamine (0.12mol) and 12.2g of triethylamine (0.12mol) were dissolved in 150mL of tetrahydrofuran, and the temperature was controlled below 40°C and added dropwise to the reaction system, and reacted at room temperature for 2 hours. The temperature was raised to 40° C. and stirring was continued for 15 minutes, and the starting material basically disappeared as monitored by TLC.
[0028] S2: Cool the system to 5°C overnight, then filter, add 50...
Embodiment 2
[0031] S1: Stir and dissolve 36.3g of n-hexadecanol (0.15mol) in 150mL of tetrahydrofuran, then add 16.8g of triethylamine (0.165mol), continue to stir well, and dissolve 27.6g of phosphorus oxychloride (0.18mol) Into 15mL of tetrahydrofuran, then lower the temperature to below 15°C, and slowly add it dropwise to the reaction system, controlling the temperature not to exceed 20°C. After the drop is complete, stir and react at 20°C for 2 hours, and the raw materials basically disappear under TLC monitoring. Then 16g of dimethylethanolamine (0.18mol) and 18.3g of triethylamine (0.18mol) were dissolved in 225mL of tetrahydrofuran, and the temperature was controlled below 40°C and added dropwise to the reaction system, and reacted at room temperature for 2 hours. The temperature was raised to 40° C. and stirring was continued for 15 minutes, and the starting material basically disappeared as monitored by TLC.
[0032] S2: Cool the system to 5°C overnight, then filter, add 75mL of ...
Embodiment 3
[0035] S1: Stir and dissolve 242g of n-hexadecanol (1mol) in 1000mL of tetrahydrofuran, then add 112g of triethylamine (1.1mol), continue to stir well, and dissolve 184g of phosphorus oxychloride (1.2mol) into 100mL of tetrahydrofuran Then lower the temperature to below 15°C, slowly add dropwise to the reaction system, control the temperature not to exceed 20°C, stir and react at 20°C for 2 hours after dropping, TLC monitors that the raw materials basically disappear. Then 107g of dimethylethanolamine (1.2mol) and 122g of triethylamine (1.2mol) were dissolved in 1500mL of tetrahydrofuran, and the temperature was controlled below 40°C and added dropwise to the reaction system, and reacted at room temperature for 2 hours. The temperature was raised to 40° C. and stirring was continued for 15 minutes, and the starting material basically disappeared as monitored by TLC.
[0036] S2: Cool the system to 5°C overnight, then filter, add 500mL of 2mol / L hydrochloric acid (1mol) dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com