Hyaluronic acid skin protection composition and preparation method and application thereof
A hyaluronic acid and skin protection technology, applied in the field of biomedicine, can solve the problems of ineffective removal of active oxygen free radicals, achieve good skin protection effects, inhibit oxidative stress, and improve skin aging symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
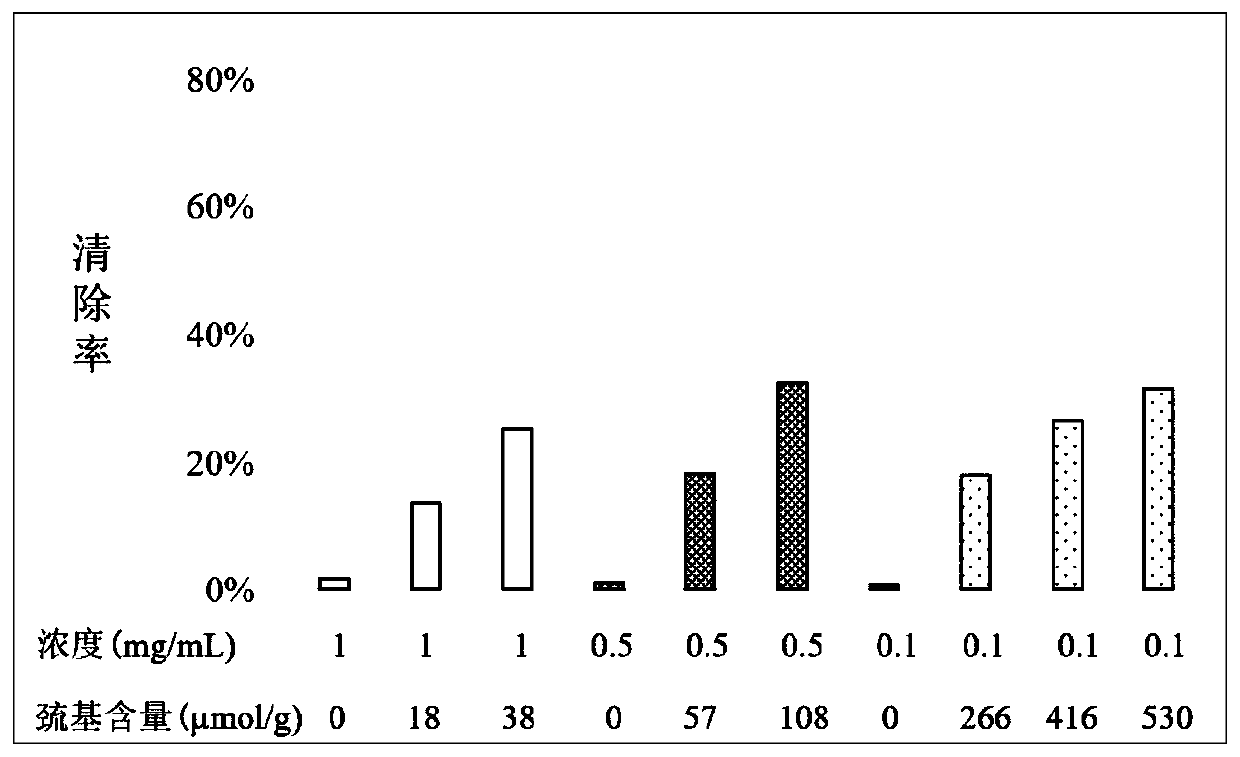
[0048] Example 1: Antioxidative effect of hyaluronic acid thiolated derivatives with different thiol content in scavenging free radicals
[0049] The 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH) test method was used to evaluate the free radical scavenging performance of the hyaluronic acid mercapto derivatives used in the present invention. DPPH is widely used in the quantitative determination of the antioxidant capacity of biochemical substances; this method is based on the fact that the DPPH free radical has a single electron and has a strong absorption at a wavelength of 517nm, and its alcohol solution is purple; when there is a free radical scavenger At the same time, the absorption gradually disappears due to the pairing with its single electron, and its fading degree is quantitatively related to the number of electrons it accepts, so a spectrophotometer can be used for rapid quantitative analysis (Sharma et al., Food Chemistry 2009, 113: 1202-1205).
[0050] Hyaluronic ...
Embodiment 2
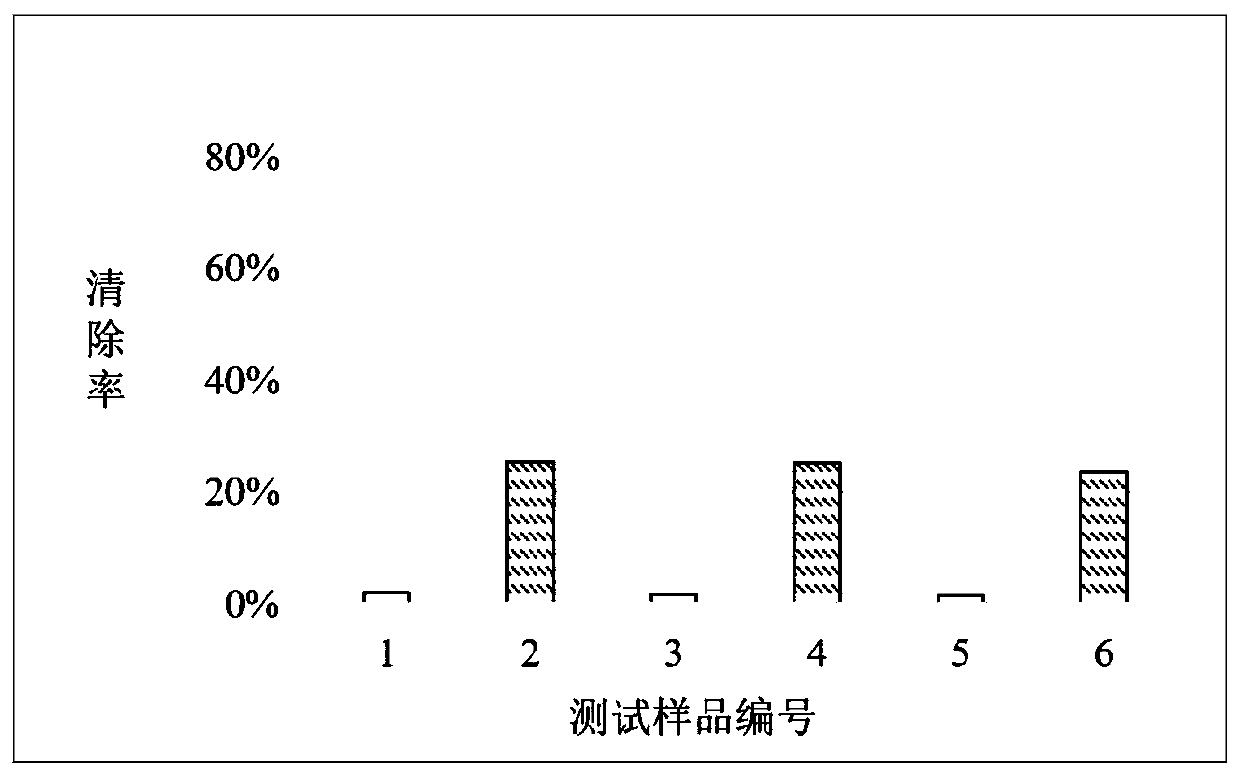
[0057] Example 2: Antioxidative effect of hyaluronic acid mercapto derivatives with different molecular weights in scavenging free radicals
[0058] Hyaluronic acid thiol derivatives are prepared from sodium hyaluronate of 180KDa, 300KDa and 1,500KDa respectively by the method reported by Shu et al. 38 μmol / g, 33 μmol / g and 40 μmol / g.
[0059] The sodium hyaluronate raw material and the above-mentioned sodium hyaluronate mercapto derivatives were respectively dissolved in a phosphate buffer solvent (pH7.4) to obtain a test sample solution as described in the following table.
[0060]
[0061] According to the DPPH method described in Example 1, the antioxidant effect of the above sample solution in scavenging free radicals was measured.
[0062] The results of this example can be found in figure 2 . Test samples 1, 3 and 5 are sodium hyaluronate control solutions of different molecular weights, and the DPPH free radical scavenging rate is low; Test samples 2, 4 and 6 ar...
Embodiment 3
[0063] Embodiment 3: Preparation of hyaluronic acid skin protection composition (containing sodium hyaluronate of a molecular weight)
[0064] The thiolated derivatives of hyaluronic acid used were prepared from 180KDa sodium hyaluronate using the method reported by Shu et al. , 38 μmol / g, 57 μmol / g, 108 μmol / g, 266 μmol / g, 416 μmol / g and 530 μmol / g.
[0065] The molecular weight of sodium hyaluronate used is 1.6KDa, 3KDa, 8KDa, 50KDa, 180KDa, 500KDa, 800KDa, 1,500KDa, 2,700KDa.
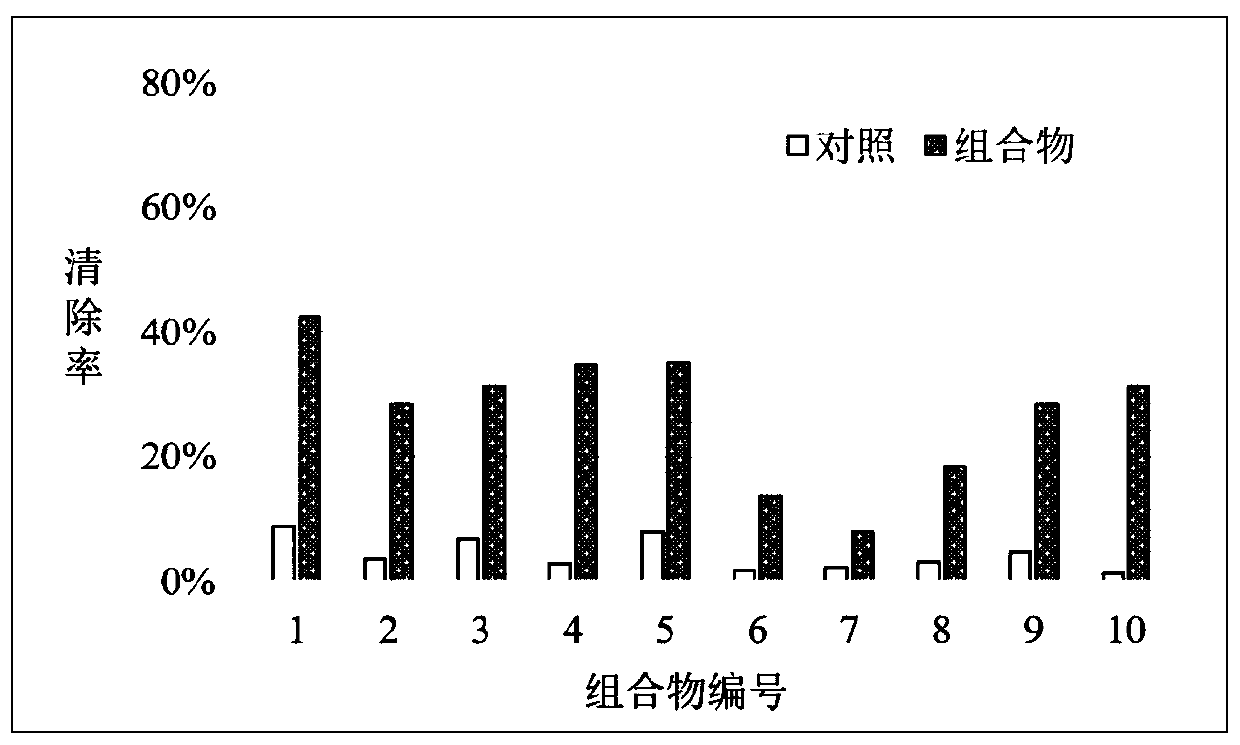
[0066] Any of the above-mentioned hyaluronic acid thiolated derivatives with a thiol content was dissolved in distilled water to obtain a 6 mg / mL solution; any of the above-mentioned sodium hyaluronate with a molecular weight was dissolved in distilled water to obtain a 12 mg / mL solution; then according to 0.9: The volume ratio of 0.1-0.1:0.9 is evenly mixed to obtain hyaluronic acid skin protection compositions with different proportions. In these compositions, the content of hyaluronic acid merca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com