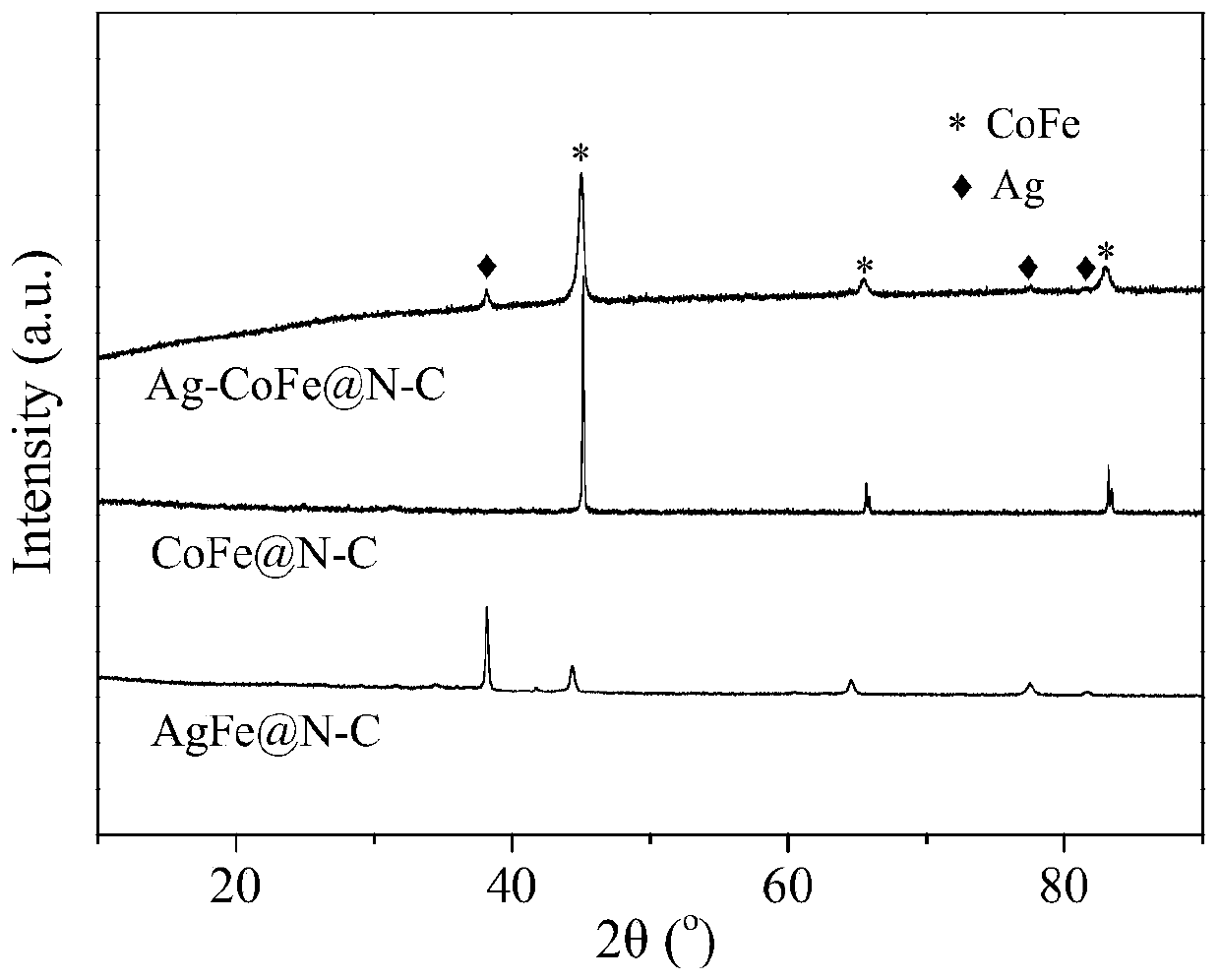
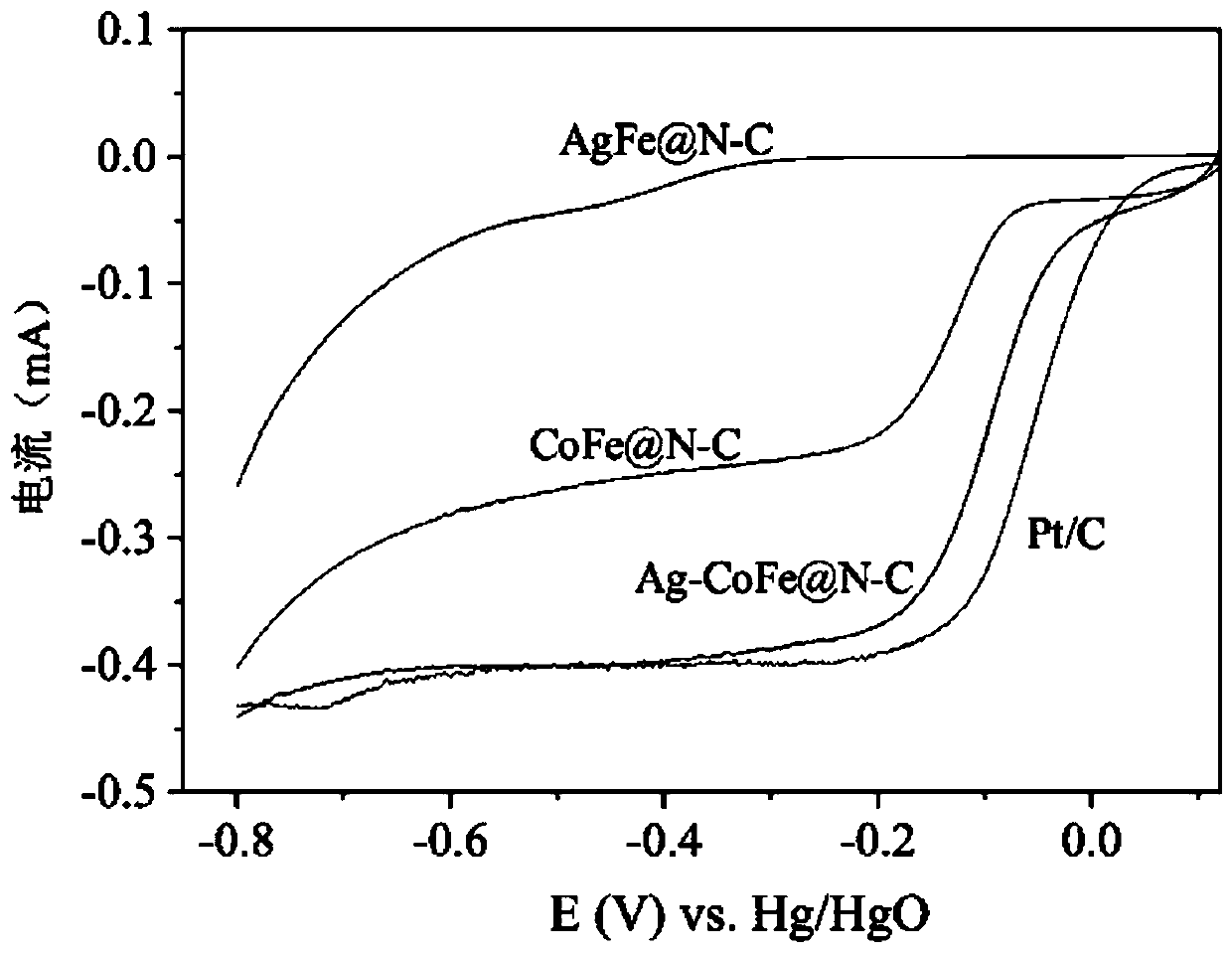
Preparation method of novel transition metal-nitrogen co-doped carbon material oxygen reduction/oxygen evolution difunctional catalyst
A bifunctional catalyst, transition metal technology, applied in physical/chemical process catalysts, chemical instruments and methods, electrolysis processes, etc., to achieve the effects of avoiding agglomeration, low cost, and high dopant content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A method for preparing a transition metal-nitrogen co-doped carbon material oxygen reduction / oxygen evolution bifunctional catalyst according to an embodiment of the present invention comprises the following steps:
[0029] mixing cobalt salt, surfactant and deionized water to prepare cobalt salt solution;
[0030] Under the condition of stirring, mix the iron salt solution with the cobalt salt solution, stir and react for 0.5h to 1.0h, and obtain the first mixed solution, wherein the iron salt solution is potassium ferricyanide solution;
[0031] Mix the silver salt aqueous solution with the first mixed solution under stirring conditions, and stir and react for 10.0h to 14.0h to prepare the second mixed solution;
[0032] Filter the second mixed solution, wash the filter residue and dry at 70°C to 90°C for 10.0h to 14.0h to obtain a catalyst precursor;
[0033] The catalyst is calcined at 500°C to 900°C for 1.0h to 3.0h in an inert atmosphere to prepare a transition m...
Embodiment 1
[0047] Dissolve 0.01mol of cobalt nitrate and 0.05mol of surfactant in deionized water to 1000mL to obtain a cobalt salt solution; dissolve 0.01mol of potassium ferricyanide to 1000mL in deionized water to obtain an iron salt Aqueous solution: Dissolve 0.01 mol of silver nitrate in deionized water to a volume of 1000 mL to obtain a silver salt solution.
[0048] Under the condition of magnetic stirring, add 70mL of iron salt solution dropwise into 100mL of cobalt salt solution, continue to stir for 45min, then add 10mL of silver salt solution dropwise into the above mixture under stirring, continue to stir for 12h , filtered, and the resulting filter residue was washed with ethanol and deionized water in sequence, and dried at 80° C. for 12 hours to obtain a catalyst precursor.
[0049] Grind the catalyst precursor into powder, take a certain amount of catalyst precursor and put it in a porcelain boat in a tube furnace, raise the temperature to 700°C at 5.0°C / min in an inert g...
Embodiment 2
[0051] Dissolve 0.01mol of cobalt nitrate and 0.01mol of surfactant in deionized water to 1000mL to obtain cobalt salt solution; dissolve 0.01mol of potassium ferricyanide to 1000mL in deionized water to obtain iron salt Aqueous solution: Dissolve 0.01 mol of silver nitrate in deionized water to a volume of 1000 mL to obtain a silver salt solution.
[0052] Under the condition of magnetic stirring, add 70mL of iron salt solution dropwise into 100mL of cobalt salt solution, continue to stir for 45min, then add 10mL of silver salt solution dropwise into the above mixture under stirring, continue to stir for 12h , filtered, and the resulting filter residue was washed with ethanol and deionized water in sequence, and dried at 80° C. for 12 hours to obtain a catalyst precursor.
[0053] Grind the catalyst precursor into powder, take a certain amount of catalyst precursor and put it in a porcelain boat in a tube furnace, raise the temperature to 500°C at 0.5°C / min in an inert gas at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com