SN-38-PLGA sustained release microsphere for intratumoral injection and preparation method and application thereof
A technology of intratumoral injection and sustained-release microspheres, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, microcapsules, etc. Poor drug resistance, loss of anti-tumor activity, etc., to achieve good application prospects, improve drug utilization, and reduce the number of administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0058] Another specific embodiment of the present invention, in the step (1),
[0059] The mass ratio of SN-38 to PLGA (i.e. drug-to-lipid ratio, w / w) is 1:5-20 (preferably 1:10);
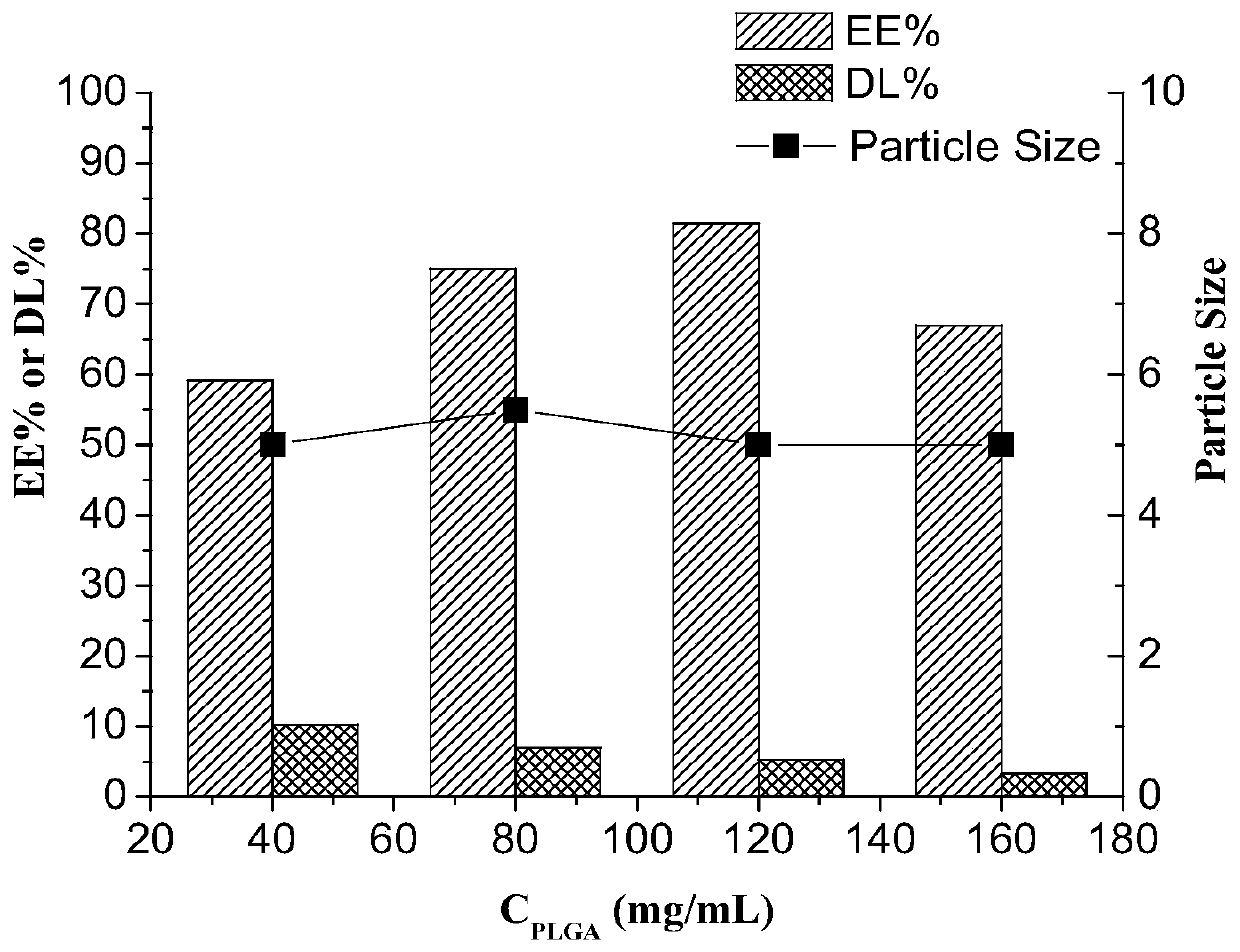
[0060] The concentration of the PLGA is 40~160mg / mL (preferably 80mg / mL);
[0061] Another specific embodiment of the present invention, in the step (2),
[0062] The volume ratio of the oil phase to the water phase in the colostrum is 1:1 to 8 (preferably 1:3);
[0063] The polyvinyl alcohol concentration in the water phase is 0.5-2% (preferably 1.5%);
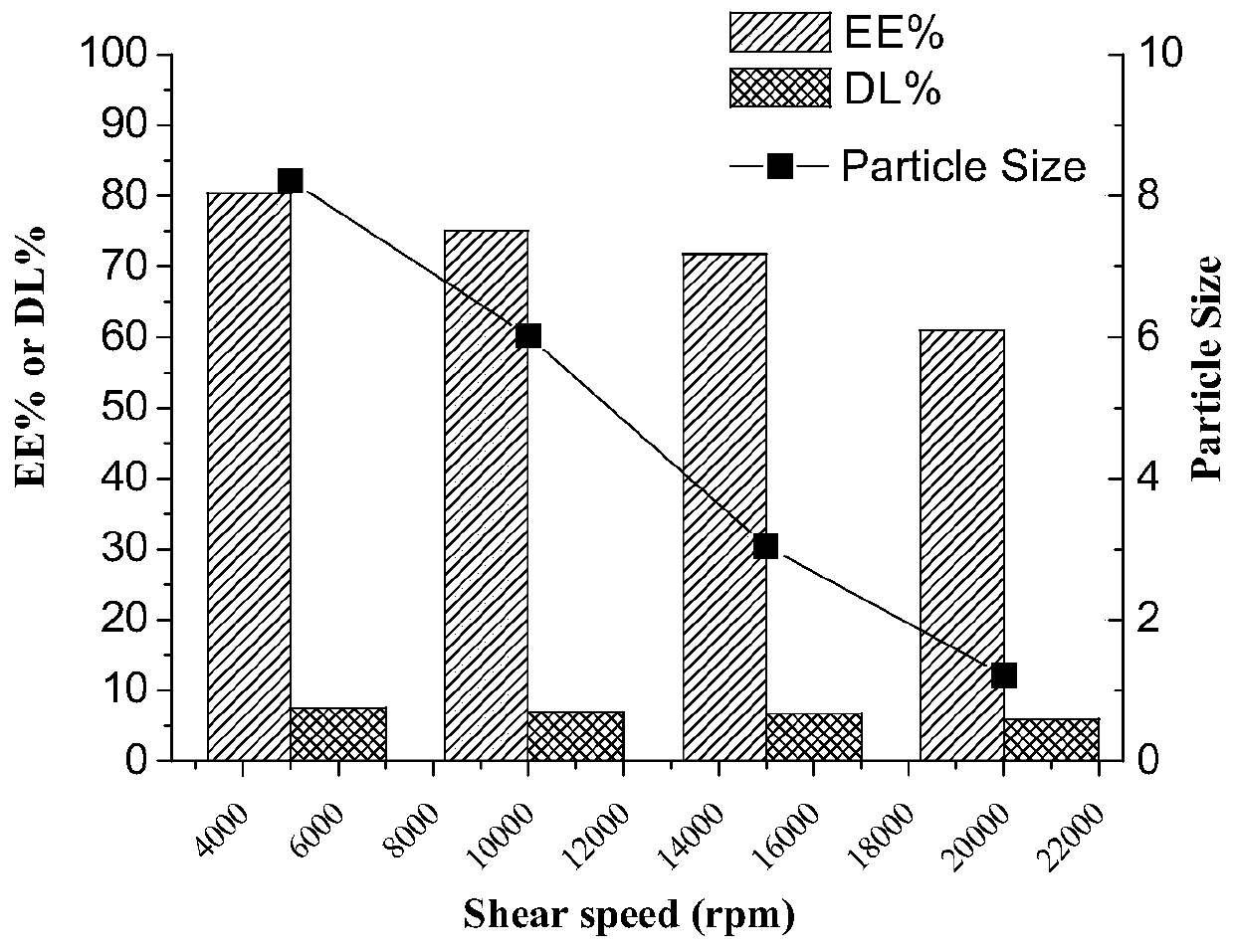
[0064] The shear rate is controlled to be 5000~20000r / min (preferably 5000r / min);
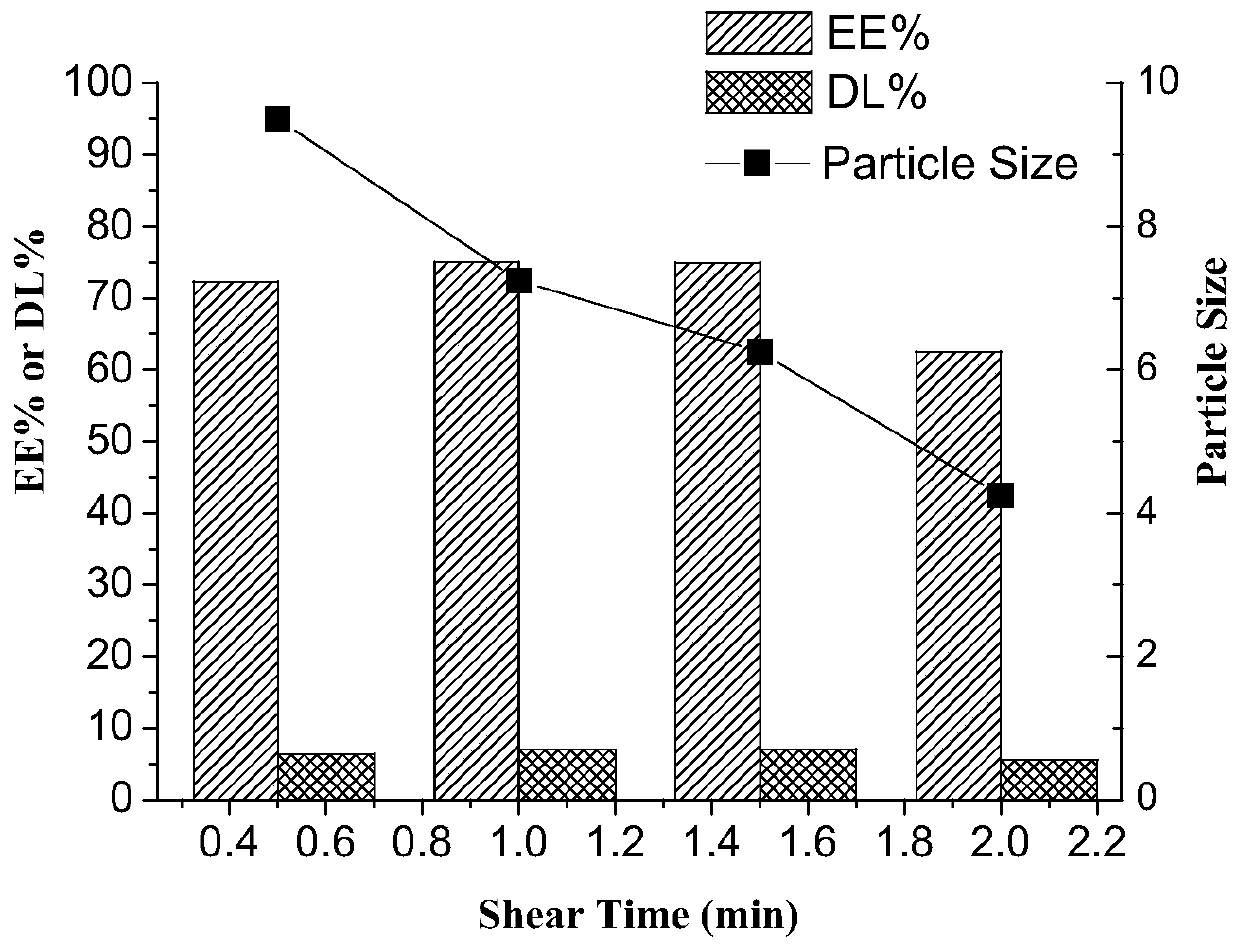
[0065] Shearing time is 0.5~2min (preferably 1min);
[0066] Another specific embodiment of the present invention, in the step (3),
[0067] The volume ratio of the aqueous phase to the dispersed phase in the colostrum is 1:3 to 10 (preferably 1:5);
[0068] The polyvinyl alcohol concentration in the dispersed phase is 0.1 to 1.0% (preferably 0.5%),
[0069]...
Embodiment 1
[0078] 1 Experimental materials
[0079] 1.1 Drugs and reagents
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com