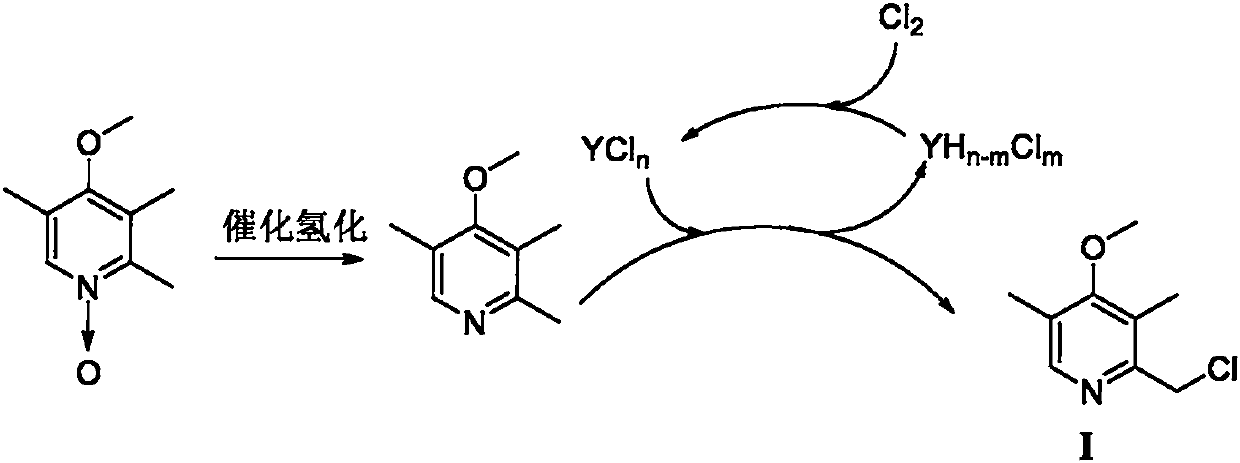
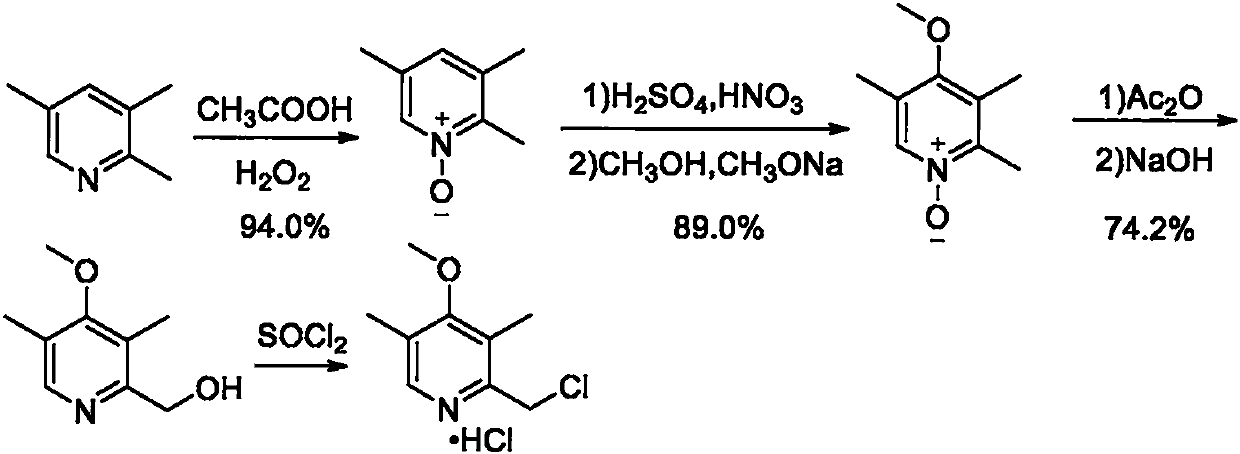
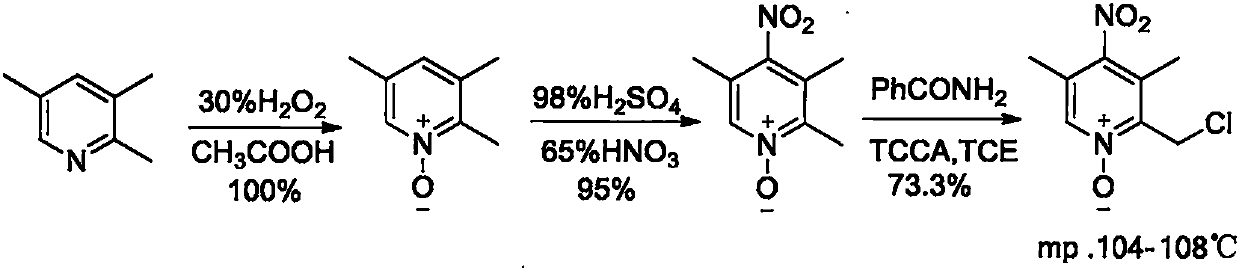
Preparation method of omeprazole midbody
A technology of chloromethyl and methoxypyridine, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as fire, corrosion, and flammability, and achieve high yield, mild reaction conditions, and high environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of Raney Ni
[0051]
[0052] Add 5g (12.5mmol) NaOH, heat 25ml water to 50°C and stir, after 10min, add 4.5g aluminum-nickel alloy in small amounts (0.2~0.4g) to 20% sodium hydroxide solution in batches, and finish adding aluminum within 1h -After the nickel alloy, stir for 4 hours; add 20ml of water and stir for 30min, wash three times, and wash with ethanol (3×20ml) to obtain Raney Ni, which spontaneously ignites in air (deactivation after long-term storage), and is directly used in the reduction reaction.
Embodiment 2
[0054] Synthesis of 2,3,5-trimethyl-4-methoxypyridine
[0055]
[0056] At room temperature, 20.0g (0.12mol) 2,3,5-trimethyl-4-methoxypyridine-N-oxide, add 2.0g (10wt.%) Raney Ni prepared in Example 1, 200ml methanol In the reaction flask; after the air was pumped out, hydrogen gas was introduced, and the temperature was raised to 60°C and the reaction was stirred for 6h. After the reaction, filter with suction, spin off methanol, add 50ml of dichloromethane, extract with saturated brine (3×50ml), dry the organic layer with anhydrous sodium sulfate, remove the solvent, and dry to obtain 17.5g of a colorless transparent oily liquid 2,3,5-Trimethyl-4-methoxypyridine, yield 96.8%. 1 H NMR (400MHz, CDCl 3 )δ: 2.15(s, 3H, CH 3 ), 2.18(s, 4H, CH 3 ), 2.42 (s, 4H, CH 3 ), 3.70 (s, 3H, OCH 3 ), 8.08 (s, 1H, pyridine ring-H).
Embodiment 3
[0058] Preparation of 2,3,5-trimethyl-4-methoxypyridine
[0059]
[0060] Add 90mmol 2,3,5-trimethyl-4-methoxypyridine-N-oxide, 1.5g Raney nickel and 200ml ethyl acetate into a 500ml flask, feed hydrogen, stir at 40°C for 5.0h; TLC monitoring After the reaction, after the reaction was completed, suction filtration, precipitation, separation and purification gave 13.12 g of brown liquid 2,3,5-trimethyl-4-methoxypyridine, with a yield of 96.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com