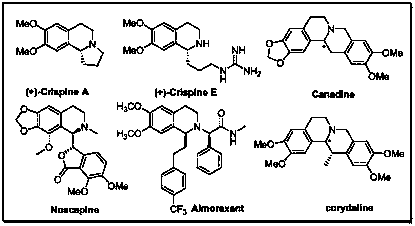
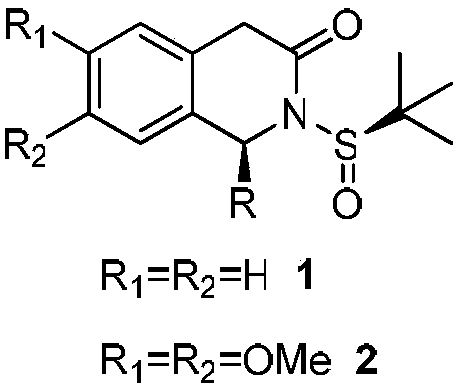
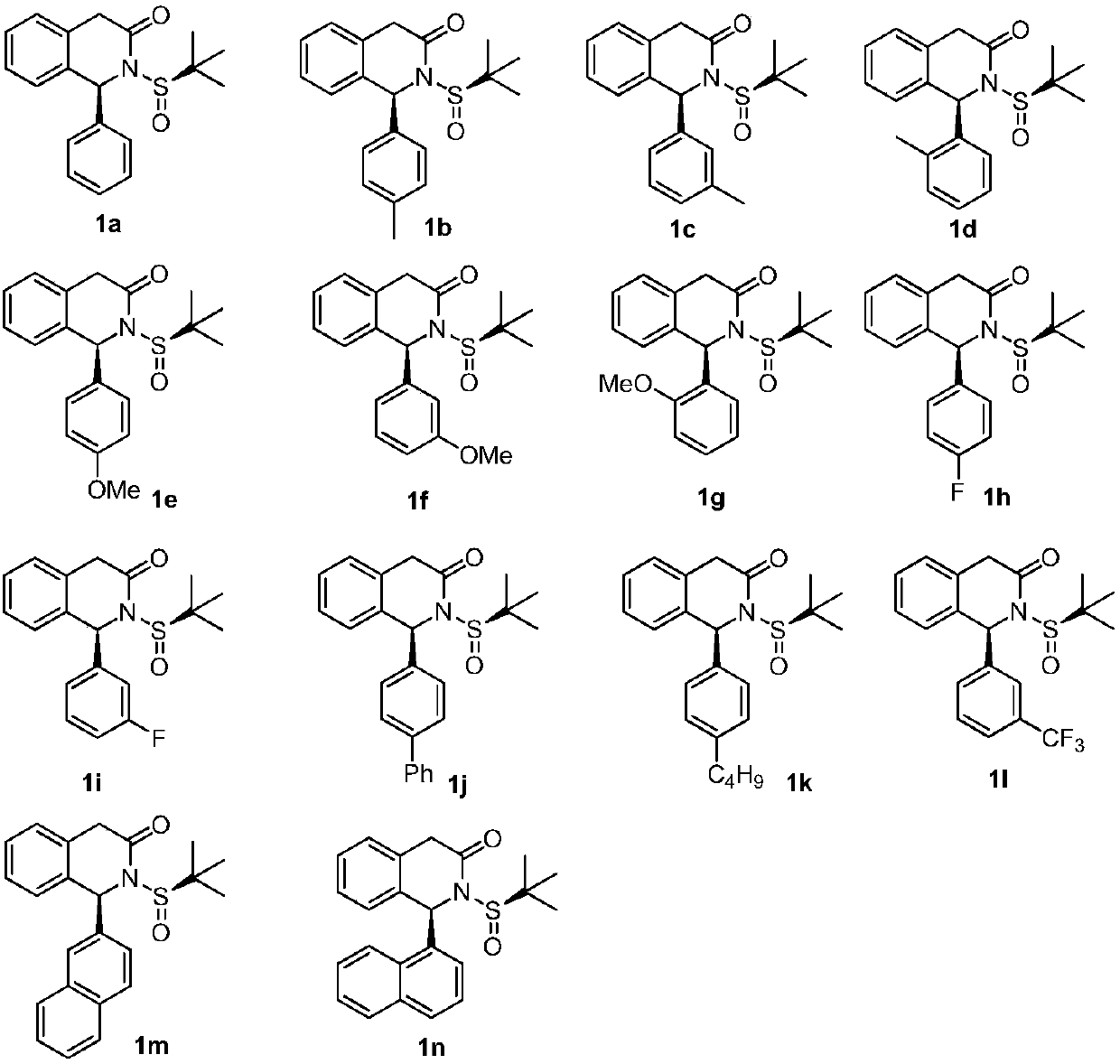
1-substituted isoquinoline ketone compound and preparation method thereof
A technology for isoquinolinones and compounds, applied in the field of 1-substituted isoquinolinone compounds and their preparation, can solve problems such as lack of universality, and achieve the effects of simple route, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of compound 1a
[0026] Add phenylmagnesium bromide (5.3mL, 5.33mmol, 1M in THF) to 2,2'-bipyridine (833mg, 5.33mmol) in dichloromethane (15mL) under argon protection and ice-salt bath and stir for 15 Minutes, the dichloromethane solution (5mL) of compound 3 (500mg, 1.78mmol) was dripped into the reaction system, and after reacting for 1 hour, a saturated aqueous ammonium chloride solution was added, extracted three times with dichloromethane, and the organic phase was washed with saturated brine , dried, concentrated and purified by silica gel column to obtain white solid compound 1a (430 mg, 74%). 1 H NMR (400 MHz, CDCl 3 )δ7.53-7.49(m,1H),7.40-7.36(m,2H),7.34-7.28(m,2H),7.27-7.18(m,3H),7.12-7.08(m,1H),6.19( s,1H),3.90(d,J=18.8Hz,1H),3.68(d,J=18.8Hz,1H),1.15(s,9H)ppm.
[0027]Synthesis of compound 1b
[0028] 4-Methylphenylmagnesium bromide (5.3mL, 5.33mmol, 1M in THF) under argon protection and ice-salt bath
[0029] Add 2,2'-bipyridine (833mg, 5.33mmo...
Embodiment 2
[0067] The preparation of compounds 1b-n, 2b-2f was the same as in Example 1.
[0068] Synthesis of compound 1a
[0069]Add phenylmagnesium bromide (5.3mL, 5.33mmol, 1M in THF) to N-methylmorpholine (1.2mL, 10.66mmol) in dichloromethane (15mL) under argon protection and ice-salt bath and stir for 15 Minutes, the dichloromethane solution (5mL) of compound 3 (500mg, 1.78mmol) was dripped into the reaction system, and after reacting for 1 hour, a saturated aqueous ammonium chloride solution was added, extracted three times with dichloromethane, and the organic phase was washed with saturated brine , dried, concentrated and purified by silica gel column to obtain white solid compound 1a (314mg, 54%).
[0070] Synthesis of compound 2a
[0071] Add phenylmagnesium bromide (5.3mL, 5.33mmol, 1M in THF) to N-methylmorpholine (1.2mL, 10.66mmol) in dichloromethane (15mL) under argon protection and ice-salt bath and stir for 15 Minutes, the dichloromethane solution (5mL) of compound 4 ...
Embodiment 3
[0073] The preparation of compounds 1b-n, 2a-2f was the same as in Example 1.
[0074] Synthesis of compound 1a
[0075] Add phenylmagnesium bromide (5.3mL, 5.33mmol, 1M in THF) into triethylamine (1.5mL, 10.66mmol) in dichloromethane (15mL) under argon protection and ice-salt bath and stir for 15 minutes. Compound 3 (500 mg, 1.78 mmol) in dichloromethane solution (5 mL) was dropped into the reaction system. After reacting for 0.5 hours, saturated ammonium chloride aqueous solution was added, extracted three times with dichloromethane, the organic phase was washed with saturated brine, and dried. After concentration, it was purified by silica gel column to obtain white solid compound 1a (465 mg, 80%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com