Preparation method of cariprazine
A technology of cariprazine and system, which is applied in the direction of organic chemistry, can solve the problems of unstable temperature, only 75-80%, process impurities, etc., achieve less route steps, increase yield and reaction speed, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0044] Reagents: The reactants and catalysts used in the examples of the present invention are all chemically pure and can be used directly or simply purified as needed; the organic solvents are all analytically pure and can be used directly. Reagents were purchased from China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0045] Detection instrument: high performance liquid chromatography Agilent HPLC-1260
[0046] Nuclear magnetic resonance instrument model: Bruker avance III 400
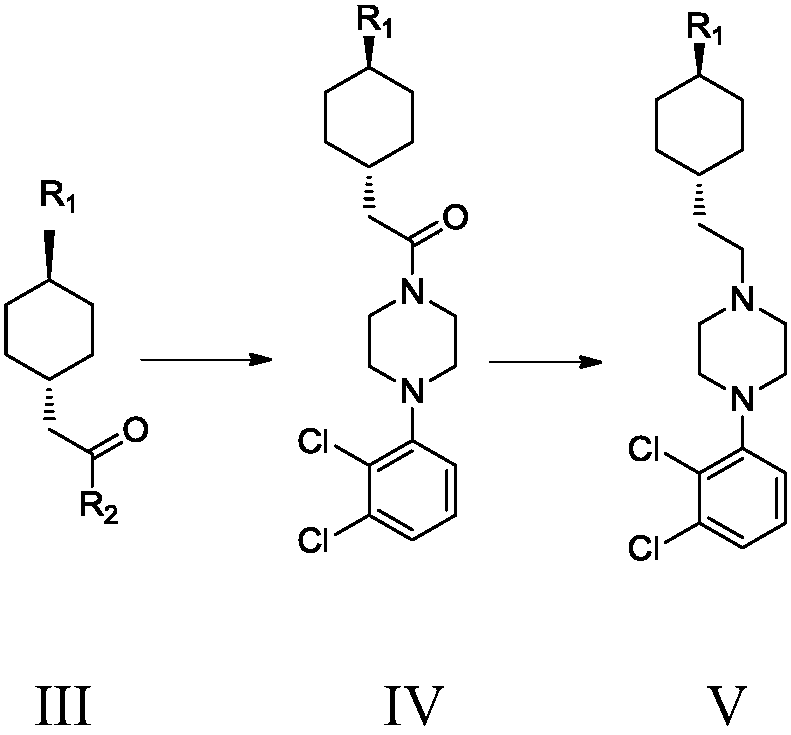
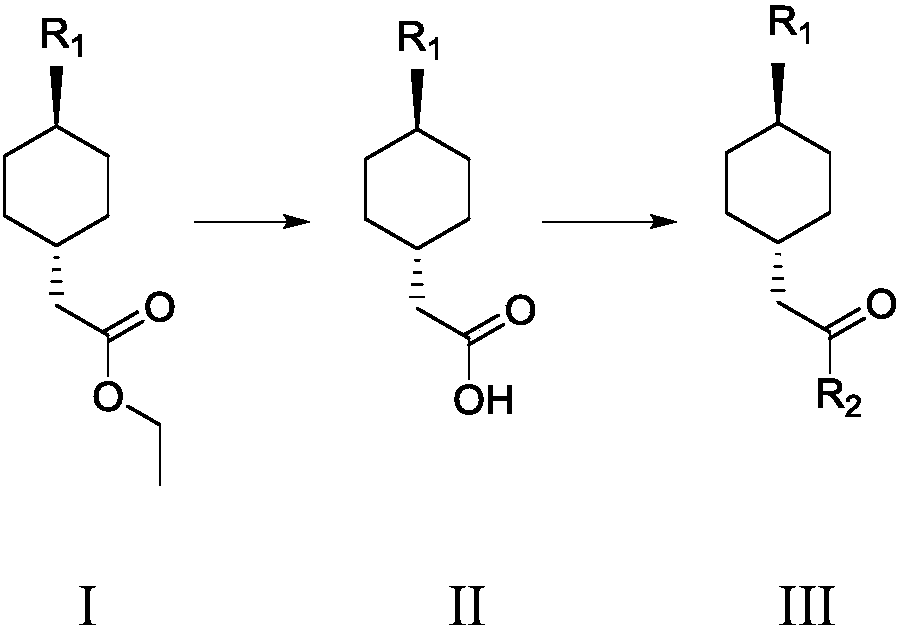
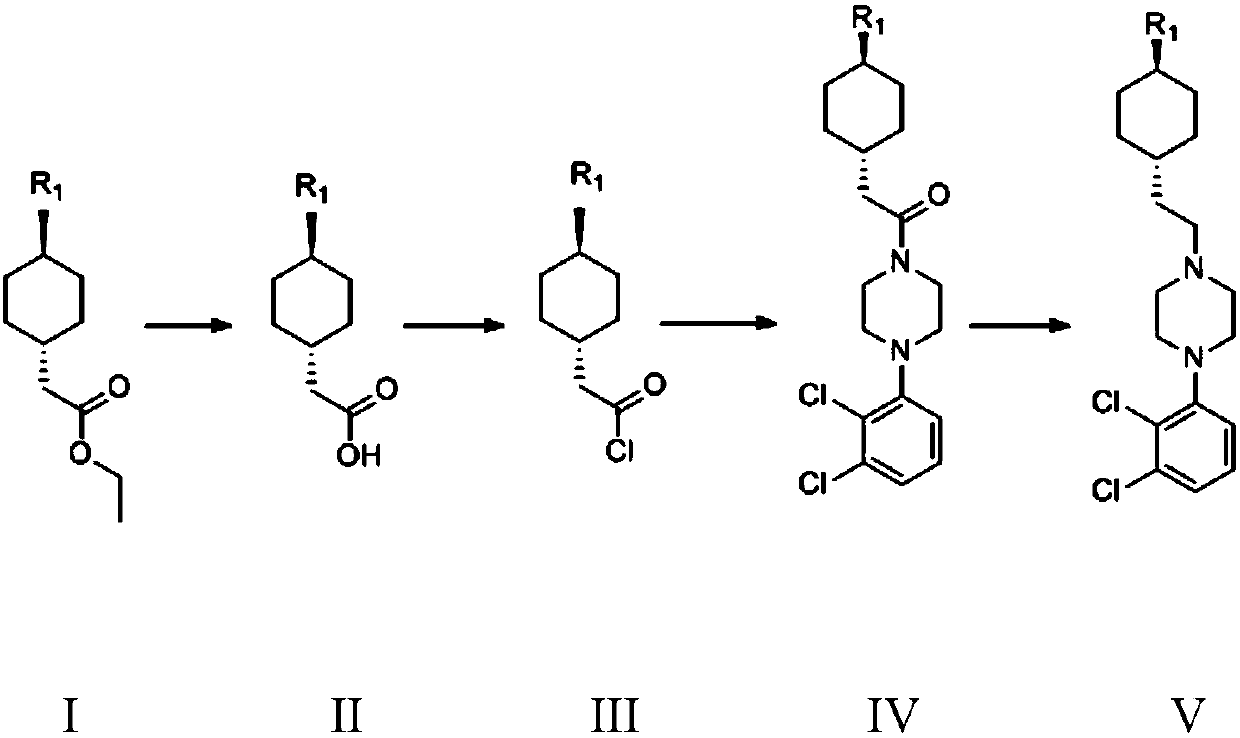
[0047] Embodiment 1 Preparation of formula III compound trans 2-(trans-4-(3,3-dimethylureido) cyclohexyl) acetyl chloride
[0048] Preparation of trans 2-(trans-4-(3,3-dimethylureido) cyclohexyl) ethyl acetate of compound of formula I
[0049] Take compound trans 2-(4-aminocyclohexyl) ethyl acetate 5.0g (0.032mol), add 50ml of dichloromethane, 30% sodium hydroxide solution 8.5g (2eq), add N,N-di Methylformyl chloride 6.9g (0.064mol), heat preservation at 30°C and stir for 5h, point...
Embodiment 5
[0059] Example 5 Formula V compound N'-[trans-4-[2-[4-(2,3-dichlorophenyl)-1-piperazinyl]ethyl]cyclohexyl]-N,N-di Preparation of methylurea
[0060] Formula IV compound 3-((trans-4-(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)-2-oxoethyl)cyclohexyl)-1,1 - Preparation of dimethylurea
[0061] Take 8.15g (0.03mol) of 1-(2,3-dichlorophenyl)piperazine hydrochloride, add 50ml of dichloromethane, add 8.3g (3eq) of potassium carbonate, cool to 0°C in an ice bath, and slowly drop into A solution composed of 5.0 g of compound III (0.02 mol) and 20 ml of dichloromethane was controlled at 0-10°C, and after 0.5 h of dripping, the plate was spotted to confirm that the reaction was complete. Add 100 ml of water to the reaction system, add hydrochloric acid, and adjust The pH value of the aqueous phase was 3-4, stirred for 10 minutes, the organic phase was separated, dried, filtered, and spin-dried to obtain 8.2 g of the compound of formula IV, with a yield of 93.1%;
[0062] Take 5g (0.011mo...
Embodiment 6
[0063] The preparation of embodiment 6 formula V compound
[0064] The preparation of formula IV compound
[0065] Take 8.15g (0.03mol) of 1-(2,3-dichlorophenyl)piperazine hydrochloride, add 50ml of dichloromethane, add 6.1g (3eq) of triethylamine, cool to 0°C in an ice bath, slowly drop Add 5.0 g of compound III (0.02 mol) and 20 ml of dichloromethane solution, control the internal temperature at 0-10 ° C, spot the plate after 0.5 h after the drop, confirm that the reaction is complete, add 100 ml of water to the reaction system, add hydrochloric acid, Adjust the pH value of the water phase to 3-4, stir for 10 minutes, separate the organic phase, dry, filter, and spin dry to obtain 8.2 g of the compound of formula IV, with a yield of 93.1%;
[0066] Take 5g (0.011mol) of the compound of formula IV, add 50ml of diethyl ether, cool the system to about 0°C, slowly drop in 28ml of DIBAL-H (1M n-hexane), keep the reaction at 0°C for 5h, and control the reaction in TLC (developing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com