Application of shikonin to preparation of rotavirus infection resisting drugs
A technology of rotavirus and shikonin, which can be used in antiviral agents, antitumor drugs, drug combinations, etc., can solve the problems of vaccine virus shedding, vaccine cost, efficacy and safety restrictions and promotion, so as to reduce the degree of diarrhea, No toxic side effects, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
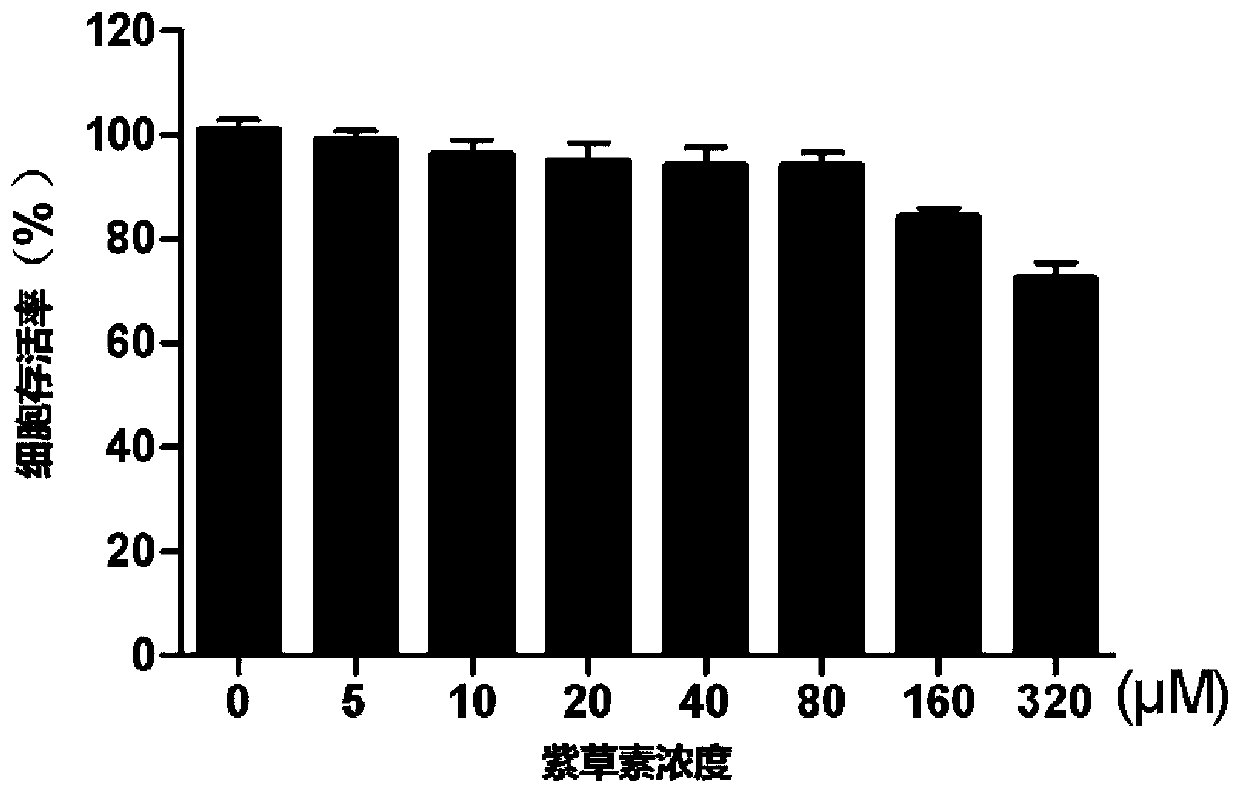
[0036] Toxicity Evaluation of Shikonin on MA104 Cells
[0037] This example studies the cytotoxicity of shikonin to normal cell MA104 cell line (monkey embryonic kidney cells). The specific operation method is: take the MA104 cell line in the exponential growth phase, and make cell suspension after trypsinization and detachment After cell counting, dilute to 1×10 with high glucose DMEM containing 10% FBS 4 / mL of cell suspension, add 100 μL of cell suspension to each well of a 96-well culture plate, each well contains about 8,000 cells; suck out the medium after the cells adhere to the wall, and add shikonin liquid with different final concentrations in each experimental group (The solvent is DMEM culture fluid), respectively 0 μM (control group), 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM, 320 μM, and the control group is the same volume of DMEM culture fluid. Set 6 parallel wells for each dose in the experimental group, and set 6 parallel wells for the control wells; place at...
Embodiment 2
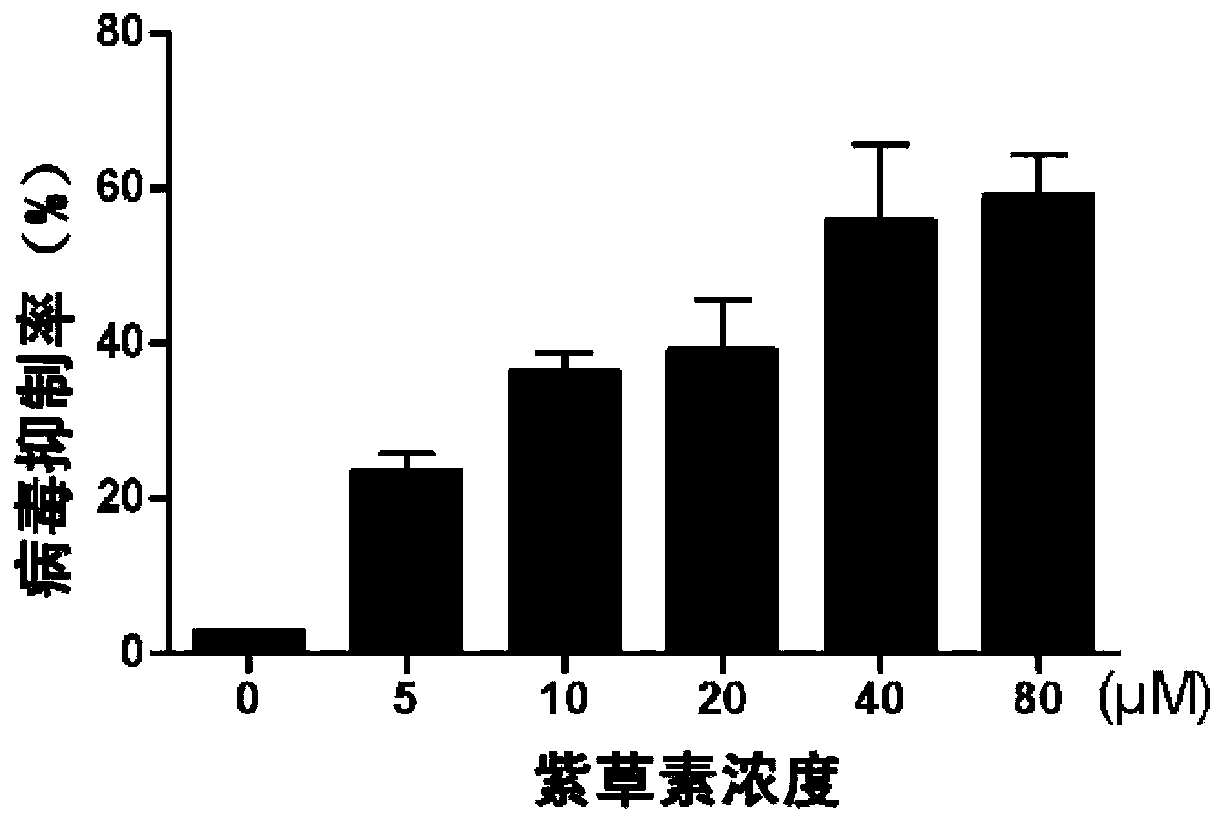
[0040] Inhibitory effect of shikonin on the replication and amplification of human rotavirus
[0041] This example studies whether shikonin has inhibitory effect on the replication and amplification of human rotavirus. 4 Cells / well were seeded in 96-well plates and incubated at 37°C for 24 h. After the cells adhered to the wall, the cell culture plate was taken out and washed once with PBS. The rotavirus solution with an MOI of 0.01 (100TCID50RV-Wa strain virus solution, donated by the Institute of Immunology, Third Military Medical University) was reacted with 10 μg / mL trypsin for 30 minutes, and then added to a 96-well culture plate, 100 μl / well. The normal cell control group was added with an equal volume of DMEM medium. All cells at 37°C, 5% CO 2 After incubation for 2 hours, the virus liquid was aspirated, and shikonin liquid of different concentrations (0 μM (virus control group), 5 μM, 10 μM, 20 μM, 40 μM, 80 μM) was added, 100 μL / well. Add an equal volume of DMEM c...
Embodiment 3
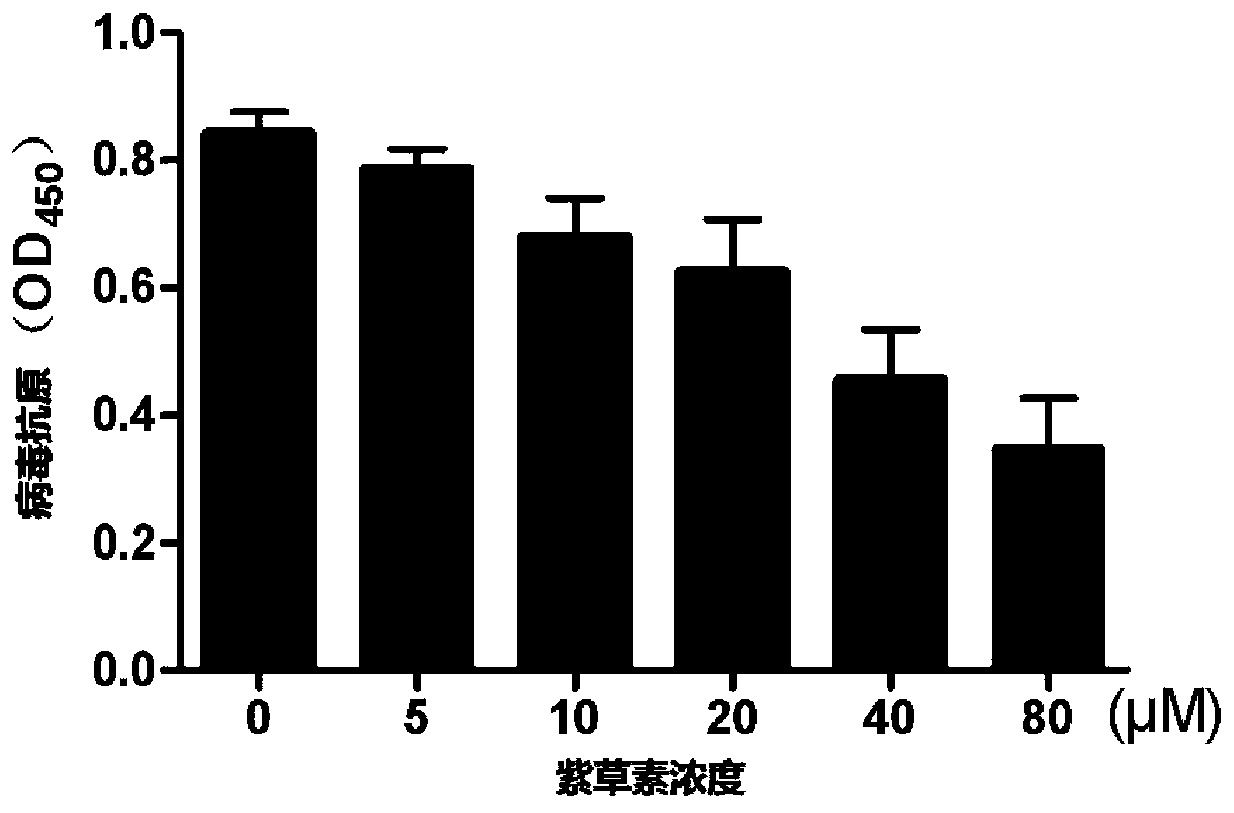
[0044] Inhibitory Effect of Shikonin on Antigen Synthesis of Human Rotavirus
[0045] This example studies whether shikonin has inhibitory effect on the synthesis of human rotavirus antigen. 5 Cell density seeded in 6-well plates at 37°C, 5% CO 2 cultivated under conditions. After the cells were completely adhered to the wall, the old culture medium in the cells was removed, and serum-free DMEM culture medium was added to the 6-well plate for 6 h. After 6 hours, the DMEM medium without FBS was aspirated, and the cells were added to 100 TCID50RV-Wa strain virus solution incubated with 10 μg / ml trypsin, and cultured at 37°C for 2 hours. After 2 hours, suck out the virus liquid, wash the cells with DMEM culture medium without FBS, add shikonin liquid at different concentrations (5 μM, 10 μM, 20 μM, 40 μM, 80 μM), and add an equal volume of DMEM without FBS to the control group Medium, continue to culture at 37°C for 24h. After 24 hours, collect the cell culture medium separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com