Preparation method and application of polypyridyl functional group modified porphyrin TTPP
A technology of functional groups and polypyridines, which is applied in the field of preparation of porphyrin TTPP modified by polypyridine functional groups, can solve the problems of cumbersome, difficult separation of synthesis methods, and inability to meet the coordination requirements of metal ions, and achieve simple process and high efficiency. High, improve the effect of relative quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
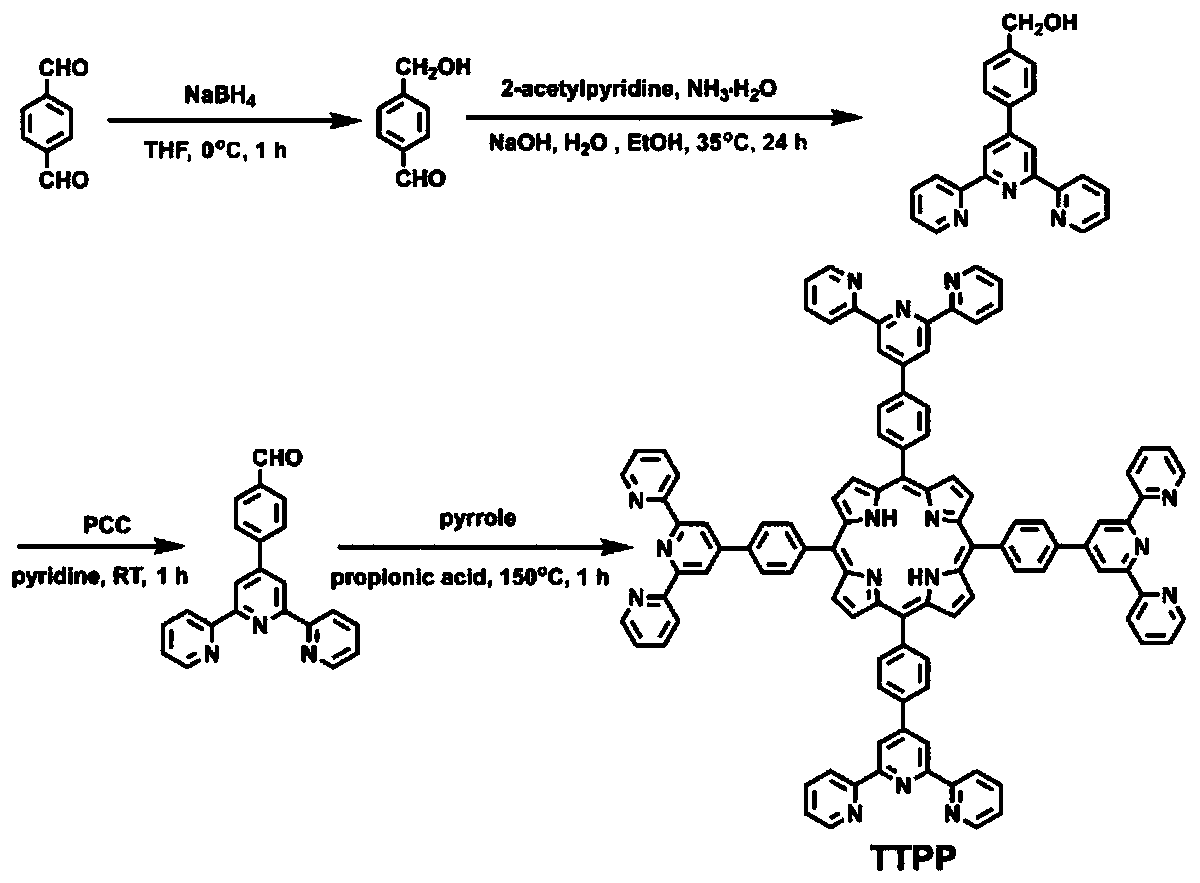
[0036] Preparation of TTPP:
[0037] Dissolve 1.7g, 5.0mmol (one equivalent) of 4'-(4-formylphenyl)-2,2':6',2'tripyridine in 50mL (per 1.0mmol 4'-(4-formyl Phenyl)-2,2':6',2'tripyridine corresponding to 10.0mL solvent) in propionic acid, reflux; 0.34mL, 5.0mmol (double equivalent) pyrrole propionic acid solution 5mL (per 1.0mmol pyrrole Corresponding to 1.0mL solvent) was slowly added dropwise, and refluxed for 1h; after the reaction system was cooled to room temperature, the reaction mixture was slowly poured into 200mL water; stood at 0°C until the precipitation was complete, and then suction filtered to obtain a purple precipitate, which was washed with methanol Slow washing to neutral (pH=7) and then slow washing with chloroform to free starting material gave the final product in 25% yield. The synthesis process of porphyrin molecule is shown in figure 1 .
[0038] For the synthesis of the porphyrin precursor 4'-(4-formylphenyl)-2,2':6',2'tripyridine, see the method de...
Embodiment 2
[0040] Assembly and application of TTPP:
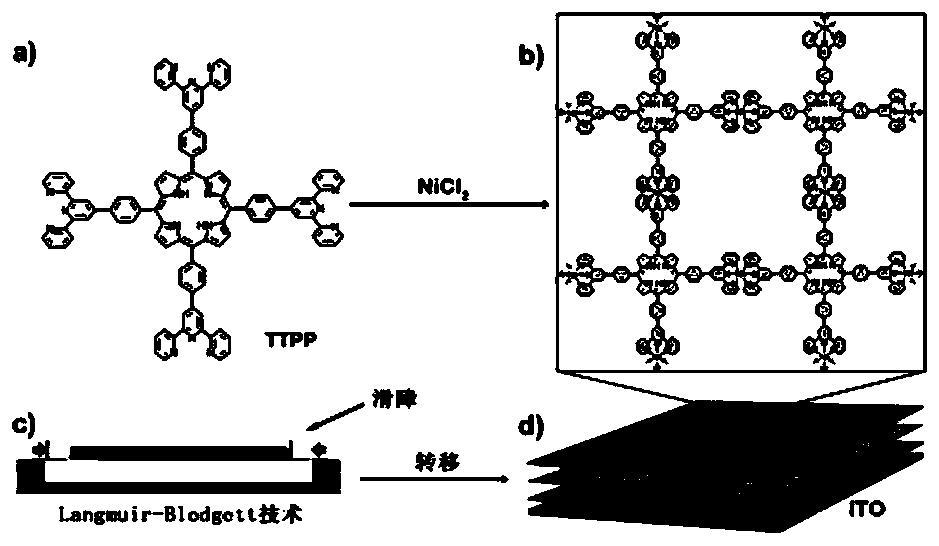
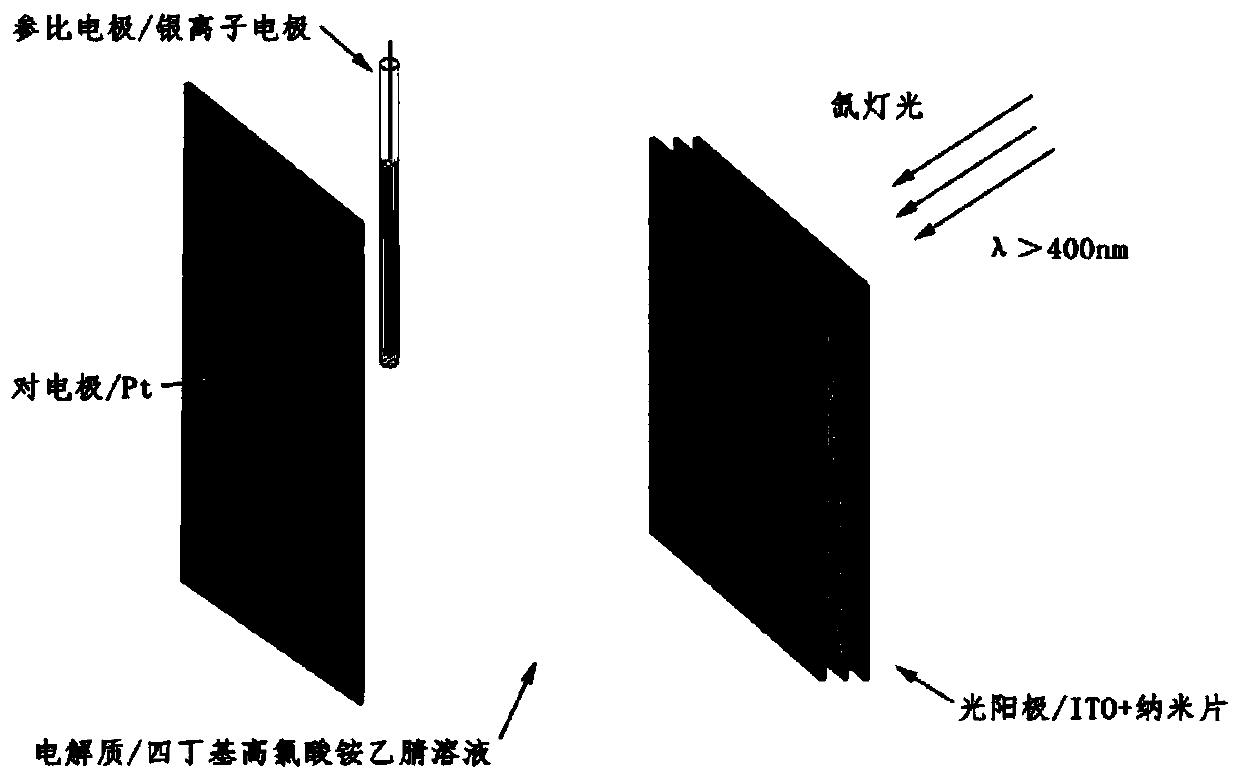
[0041] The assembly method adopts Langmuir-Blodgett technology, which can assemble porphyrin and metal ions at the gas-liquid interface. The resulting structure is a metal-organic framework (MOF) nanosheet (Ni-TTPP), which has a structure similar to single-layer graphene with large holes. The assembly structure can be used as the photoanode of solar cells to construct solar cells, and due to the ordered structure of MOF nanosheets, there are holes, and its function can be adjusted by adding guests.
[0042] The specific process of the above-mentioned Langmuir-Blodgett technique is: NiCl with a concentration of 1mM 2 The aqueous solution is a subphase, and 150 μL of TTPP chloroform solution (6.5×10 -5 M), slowly drop on the gas-liquid interface (500cm 2 ). Keep at room temperature for 30 minutes, and press the barrier at a speed of 35mm / min until the surface tension is 5mN / m. Then use ITO as the substrate, transfer the assembled n...
Embodiment 3
[0046] Experimental Study:
[0047] In order to verify the beneficial effects of the present invention, the inventor has carried out a large amount of experimental research, and part experimental process and result are as follows:
[0048] Characterization of porphyrin molecules: The synthesized porphyrin molecules were subjected to mass spectrometry, NMR, and elemental analysis to illustrate the structure and purity of the synthesized molecules. See the results at the end of the article and Figure 4 .
[0049] Structural assembly: eg figure 2 As shown, the concentration of TTPP used in the assembly experiment was 0.1mg / ml (6.5×10 -5 M) in chloroform solution. In a clean room, clean the surface of Langmuir & Langmuir Blodgett membrane analyzer with absorbent cotton soaked in ethanol or chloroform, NiCl 2 (1.0 mM) aqueous solution as a subfill analyzer tank. Then, 150 μL of 0.1 mg / ml TTPP chloroform solution (the volume of the TTPP chloroform solution taken is proportion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com