Fe nanorod, Pt@Fe nanorod catalyst, and synthesis and application
A nanorod and catalyst technology, applied in the field of preparation of Fe nanorods, can solve few problems and achieve the effects of simple synthesis method, suitable for batch scale-up, excellent catalytic performance and cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
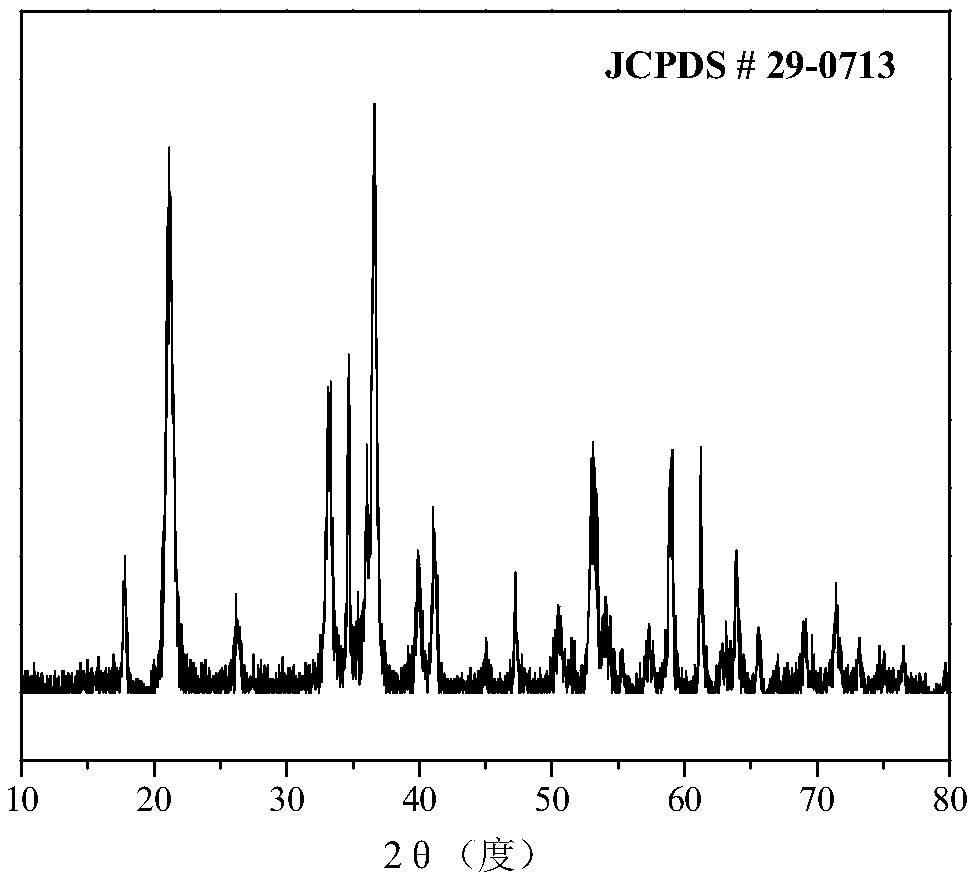
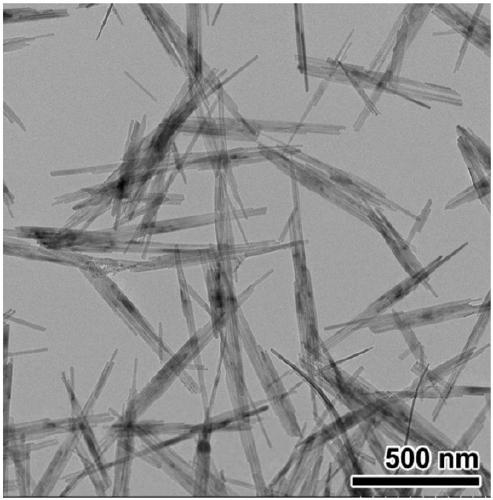
[0047] 8.1g FeCl 3 ·6H 2 O was dissolved in 80 mL of deionized water. 6.6 g of KOH (purity >85%) was dissolved in 20 mL of deionized water. After complete dissolution, the KOH solution was added dropwise to FeCl3 In the solution, a reddish-brown flocculent precipitate was gradually produced in the solution, and the process was accompanied by strong magnetic stirring. After 3 hours, the magnetic stirring was stopped, and the temperature was raised to 80° C., kept for 4 hours, and then lowered to room temperature. The resulting mixed solution was filtered and washed with deionized water and ethanol four times respectively, and after being washed to neutrality, it was dried in air for 6 hours to obtain a khaki-yellow product. XRD results see figure 1 , all diffraction peaks can be attributed to α-FeOOH (JCPDS#29-0713), which has good crystallinity. TEM results see figure 2 , the synthesized α-FeOOH has a regular nano-rod structure with a diameter of 10-20nm and a length of ...
Embodiment 2
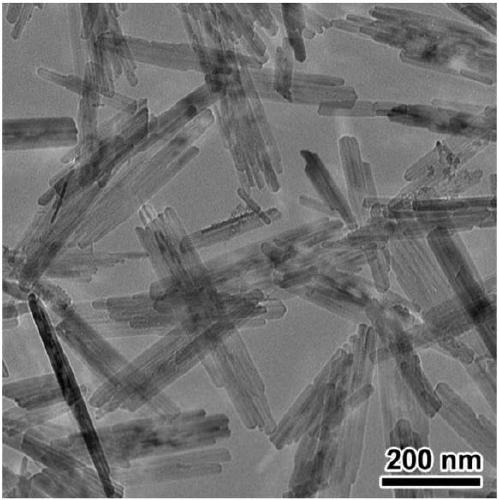
[0049] 8.1g FeCl 3 ·6H 2 O was dissolved in 80 mL of deionized water. Dissolve 3.3 g KOH in 20 mL deionized water. After complete dissolution, the KOH solution was dropped into FeCl 3 In the solution, a reddish-brown flocculent precipitate was gradually produced in the solution, and the process was accompanied by strong magnetic stirring. After 3 hours, the magnetic stirring was stopped, and the temperature was raised to 80° C., kept for 4 hours, and then lowered to room temperature. The obtained mixed solution was filtered and washed with deionized water and ethanol three times respectively, and after being washed to neutrality, it was dried in air for 6 hours to obtain a khaki-yellow product. TEM results see image 3 , the synthesized α-FeOOH has a regular nano-rod structure with a diameter of 20-30nm and a length of 200-300nm.
Embodiment 3
[0051] Ultrasonic dispersion of the α-FeOOH nanorods (27mg, 0.3mmol) obtained in Example 1 into 10mL deionized water, ultrasonication for 5min, and then under stirring, 80μL of 12mol / L concentrated HCl solution was added dropwise, and after stirring for 5min, the 5 mL of 0.8 mol / L sodium borohydride aqueous solution was added dropwise to the above solution, and reacted for 0.5 h. The black Fe nanorods were obtained, which were separated by a magnet and washed three times with water and ethanol, respectively. The electron microscope results are shown in Figure 4 , the obtained product still maintains rod-like morphology after reduction, and its size remains unchanged (diameter 10-20 nm, length 300-500 nm).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com