Acid-responsive polycationic micelle nanoparticles and preparation method and application thereof
A micellar nano and polycation technology, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, powder delivery, etc., can solve problems such as limited curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: polyethylene glycol-polyglycidyl methacrylate diblock copolymer (mPEG 113 -b-PGMA 50 -Br) preparation
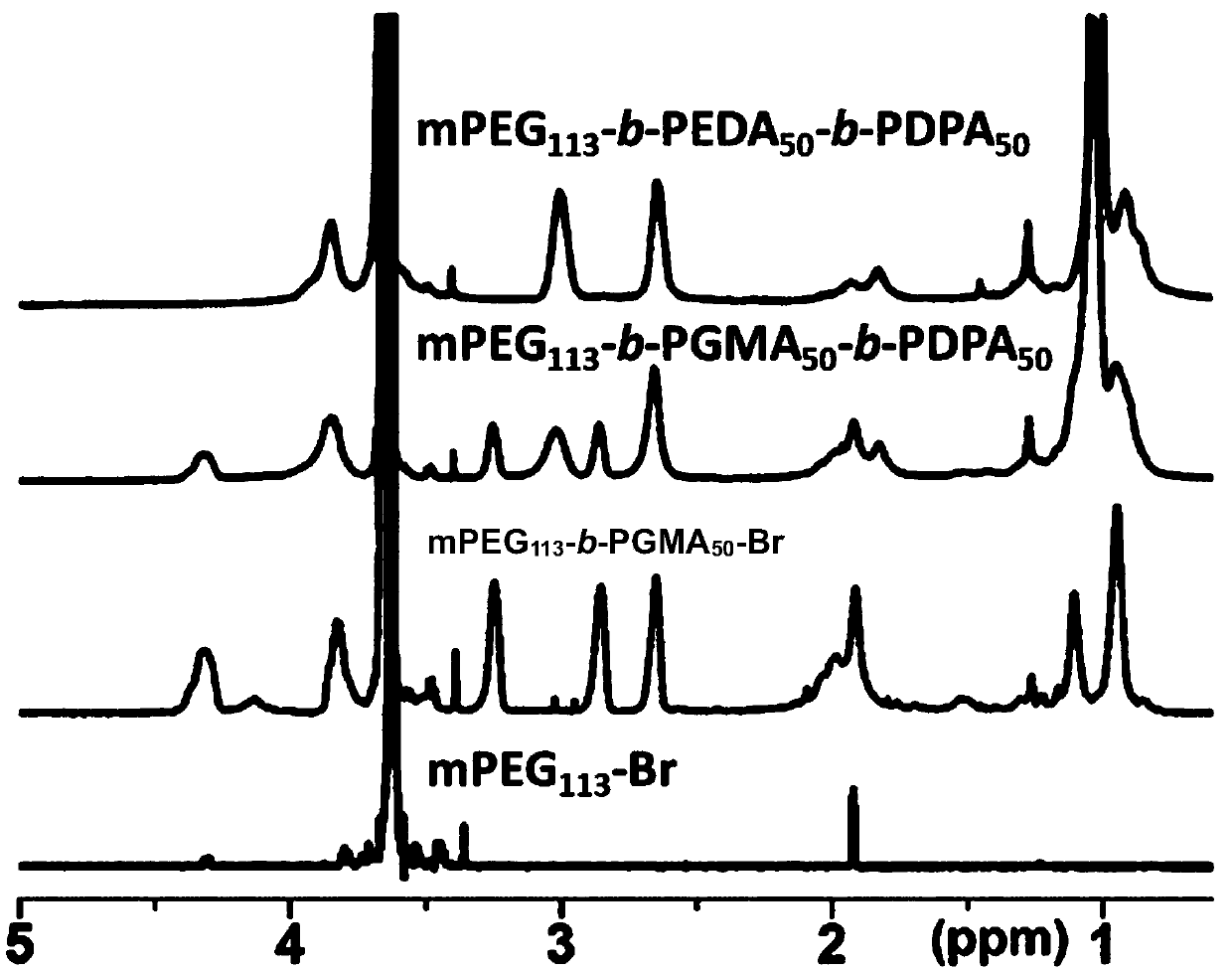
[0054] Take the polyethylene glycol macromolecular initiator (mPEG) of molecular weight 5000Da 113 -Br) 0.5 g was dissolved in 2.0 ml of N,N-dimethylacetamide (DMAC), 0.1 ml of isopropanol was added, stirred and mixed, and 1.78 ml of glycidyl methacrylate (GMA) was added, pentamethyl Diethylenetriamine 57 μl. After deoxygenation in vacuum, 7.8 mg of cuprous chloride catalyst was added and reacted at 40°C for 8 hours. After the reaction was completed, it was purified with an alumina column, concentrated by rotary evaporation, precipitated with ether, and dried with a vacuum pump to obtain 1.56 g of the product with a yield of 69%. Using deuterated chloroform as a solvent, the polymer structure was determined by H NMR spectroscopy as methoxy-terminated polyethylene glycol-polyglycidyl methacrylate (mPEG 113 -b-PGMA 50 -Br), see figure 1 .
Embodiment 2
[0055] Embodiment 2: polyethylene glycol-polyglycidyl methacrylate-polydiisopropylaminoethyl methacrylate triblock copolymer (mPEG 113 -b-PGMA 50 -b-PDPA 50 ) preparation
[0056] Get the methoxy-terminated polyethylene glycol-polyglycidylmethacrylate (mPEG) prepared in Example 2 113 -b-PGMA 50 -Br) 0.5 g was dissolved in 1.0 ml of DMAC, 0.1 ml of isopropanol was added, stirred and mixed, and then 1.31 ml of diisopropylaminoethyl methacrylate monomer and 12.6 microliters of pentamethyldiethylenetriamine were added. After deoxygenation, 6.7 mg of copper bromide catalyst was added, and reacted at 40° C. for 12 hours. After the reaction was completed, the copper salt was removed through an aluminum oxide column, concentrated by rotary evaporation at 40°C, precipitated with ether, and dried in vacuum to obtain 1.89 g of the product, with a yield of 92%. Using deuterated chloroform as a solvent, the structure of the polymer was confirmed by hydrogen nuclear magnetic resonance ...
Embodiment 3
[0057] Embodiment 3: Polyethylene glycol-ethylenediamine polyglycidyl methacrylate-polydiisopropylaminoethyl methacrylate polycation (mPEG 113 -b-PEDA 50 -b-PDPA 50 ) preparation
[0058] Get the mPEG prepared in embodiment 3 113 -b-PGMA 50 -b-PDPA 50 200mg, dissolved in 2ml DMAC. Dissolve 2.78 mL of ethylenediamine (DEA) in 3 mL of dry DMAC. The polymer solution was added dropwise to DEA, stirred and reacted at 80°C for 4 hours, then taken out, dialyzed for 24 hours with a dialysis bag with a molecular weight cut-off of 3500 Daltons (purchased from Shanghai Xinrui Biotechnology Co., Ltd.), and freeze-dried to obtain Product 113 mg, yield 62.5%, with deuterated dimethyl sulfoxide as solvent, the chemical structure of the polycation measured by proton nuclear magnetic resonance is mPEG 113 -b-PEDA 50 -b-PDPA 50 ,See figure 1 .
PUM
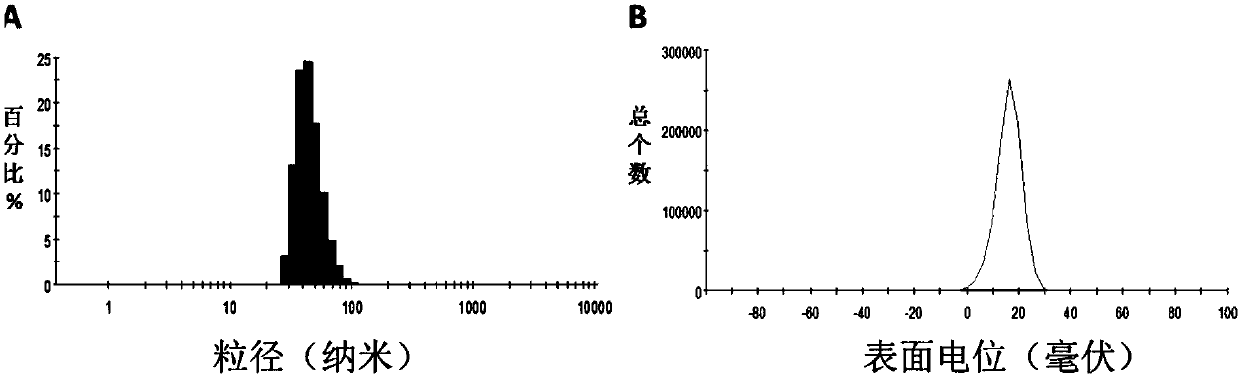
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com