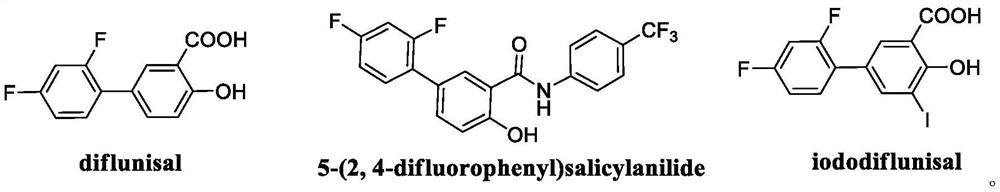
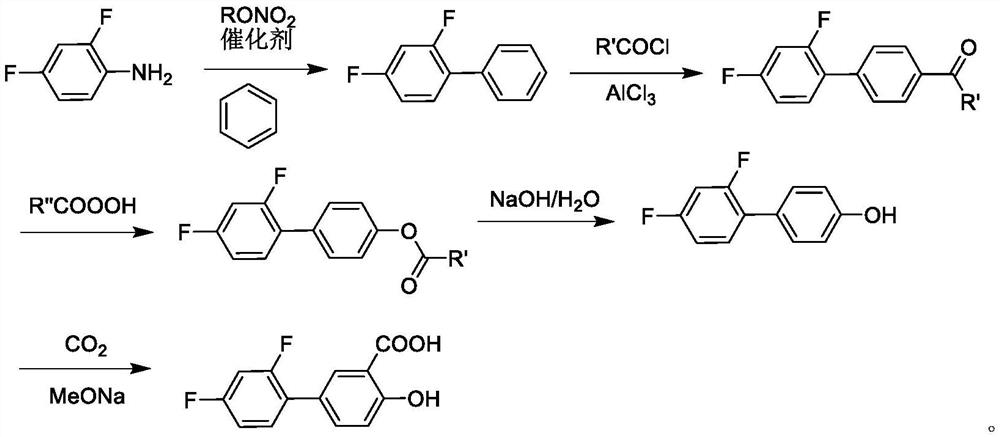
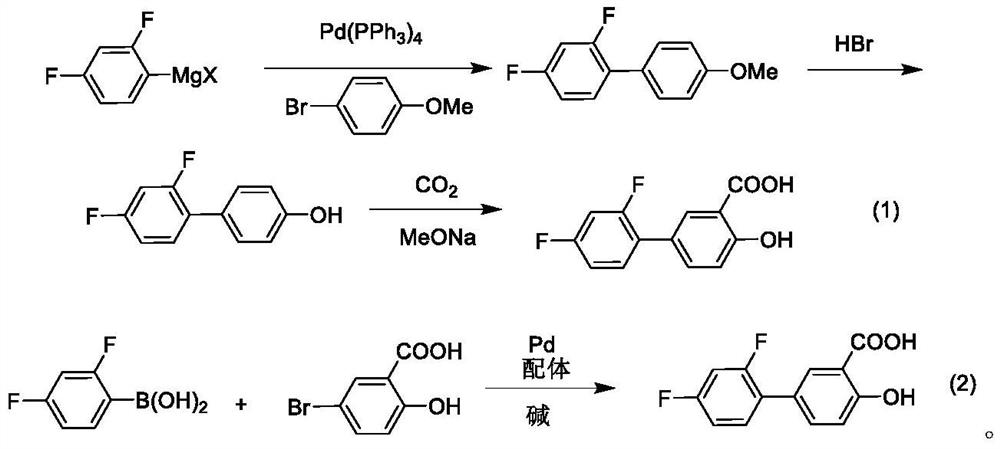
A kind of method for synthesizing diflunisal and its derivatives by one-step method
A technology for diflunisal and its derivatives, which is applied in the field of iron-catalyzed synthesis of diflunisal and its derivatives by one-step method, can solve the problems such as insufficient preparation methods of diflunisal, and achieve low cost and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1, diflunisal
[0038]
[0039] In an argon atmosphere, add Ti(OEt) into a 25mL three-neck flask 4 (456mg, 2mmol) and 2mL THF, add 17mL 2,4-difluorophenylmagnesium bromide tetrahydrofuran solution dropwise at room temperature (from 2,4-difluorobromobenzene according to the literature method [Osborne, C.A.; Endean, T.B.D.; Jarvo .E.R.Org.Lett., 2015,17,5340.]; 1M in THF, 17mmol), stirring was continued for 30min after dropping, and the resulting mixture was set aside.
[0040] Take another three-necked flask, add 5-iodosalicylic acid (1.32g, 5mmol) and 5mL THF under argon atmosphere, stir for a while, then add FeCl 3 (81.2mg, 0.5mmol), TMEDA (232mg, 2.0mmol) and 8mL toluene (THF / toluene 3:1), after stirring for about 10 minutes, slowly add the titanium reagent prepared above, and stir and reflux for 6-8 Hours, the reaction ended (TLC tracking), and dilute hydrochloric acid was added to the system until the reaction was clear. Extracted...
Embodiment 2
[0042] The preparation of embodiment 2, diflunisal
[0043]
[0044] 5-bromosalicylic acid (1.09g, 5mmol) was used to replace 5-iodosalicylic acid, and the rest of the operation was the same as above to obtain 1.04g of the product, yield: 83%.
Embodiment 3
[0045] Embodiment 3, the preparation of diflunisal
[0046]
[0047] 5-chlorosalicylic acid (863mg, 5mmol) was used instead of 5-iodosalicylic acid, and the remaining operation Example 1 was used to obtain 788mg of the product, yield: 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com